Abstract
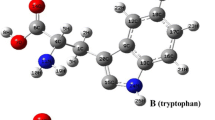
Corrosion is the deterioration of materials as a consequence of reaction to their environment. The corrosion in the instruction field poses safety, environmental threat, and financial. Based on DFT at 6‑311++G basis set and B3LYP level, calculated of several quantum chemical parameters. The three amino acids serine, tryptophan, and tyrosine have been investigated in gas and aqueous phases on corrosion of carbon steel utilizing DFT as green corrosion inhibitors. The order of inhibition efficiency in this study is serine < tyrosine < tryptophan, which relies on quantum chemical behaviors such as (LUMO) lowest unoccupied molecular orbital, (HOMO) highest occupied molecular orbital, electronegativity, bandgap energy, hardness, softness, chemical reactivity, polarization, total energy interaction of molecules, ionization energy, and electron affinity. The electrophilicity and nucleophilicity has been evaluated through Fukui indices. These are significant to estimate the most active reaction region, responsible for donating and accepting electrons to form coordinate bonds for the adsorption of the non-protonated species on the iron surface.






Similar content being viewed by others
REFERENCES
M. Dehdab, M. Shahraki, and S. M. Habibi-Khorassani, Amino Acids 48, 291 (2016).
H. M. Qadr and D. M. Mamand, J. Bio-Tribo-Corros. 7 (4), 1 (2021). https://doi.org/10.1007/s40735-021-00566-9
H. M. Qadr, Atom Indones. 46, 47 (2020).
H. Elmsellem, T. Harit, A. Aouniti, et al., Prot. Met. Phys. Chem. Surf. 51, 873 (2015).
A. Zarrouk, B. Hammouti, A. Dafali, et al., J. Saudi Chem. Soc. 18, 450 (2014).
H. M. Qadr, Ann. Dunarea de Jos Univ. Galati, Fasc. IX: Metall. Mater. Sci. 43 (4), 13 (2020).
H. M. Qadr, Phys. Part. Nucl. Lett. 18, 185 (2021).
F. Rosei, M. Schunack, Y. Naitoh, et al., Prog. Surf. Sci. 71, 95 (2003).
D. M. Mamand and H. M. Qadr, Prot. Met. Phys. Chem. Surf. 57, 943 (2021).
M. M. Solomon, S. A. Umoren, I. I. Udosoro, and A. P. Udoh, Corros. Sci. 52, 1317 (2010).
S. K. Karn, A. Bhambri, I. R. Jenkinson, et al., Corros. Rev. 38, 403 (2020).
S. K. Shukla, A. K. Singh, I. Ahamad, and M. Quraishi, Mater. Lett. 63, 819 (2009).
V. Konovalova, Mater. Today: Proc. 38, 1326 (2021).
C. Feiler, D. Mei, B. Vaghefinazari, et al., Corros. Sci. 163, 108245 (2020).
B. El Ibrahimi, A. Jmiai, L. Bazzi, and S. El Issami, Arab. J. Chem. 13, 740 (2020).
A. Al-Amiery, T. A. Salman, K. F. Alazawi, et al., Int. J. Low-Carbon Technol. 15, 202 (2020).
C. M. Fernandes, V. G. Pina, L. X. Alvarez, et al., Colloids Surf., A 599, 124857 (2020).
H. M. Qadr, Russ. J. Non-Ferr. Met. 62, 561 (2021).
D. Mamand, J. Phys. Chem. Funct. Mater. 2, 32 (2019).
D. E. Woon and T. H. Dunning, Jr., J. Chem. Phys. 103, 4572 (1995).
D. Mamand, J. Phys. Chem. Funct. Mater. 2, 77 (2019).
S. Kaya and C. Kaya, Mol. Phys. 113, 1311 (2015).
S. Kaya and C. Kaya, Comput. Theor. Chem. 1052, 42 (2015).
S. K. Saha, P. Ghosh, A. Hens, et al., Phys. E (Amsterdam, Neth.) 66, 332 (2015).
S. Kaya and C. Kaya, Comput. Theor. Chem. 1060, 66 (2015).
N. A. Kovačević and A. Kokalj, J. Phys. Chem. C 115, 24189 (2011).
S. Kaya, B. Tüzün, C. Kaya, and I. B. Obot, J. Taiwan Inst. Chem. Eng. 58, 528 (2016).
N. Obi-Egbedi, I. Obot, and M. I. El-Khaiary, J. Mol. Struct. 1002, 86 (2011).
L. M. Rodríguez-Valdez, A. Martínez-Villafañe, and D. Glossman-Mitnik, J. Mol. Struct.: THEOCHEM 713, 65 (2005).
E. Zvereva, I. Vandyukova, A. Vandyukov, et al., Russ. Chem. Bull. 61, 1199 (2012).
I. Merimi, R. Touzani, A. Aouniti, et al., Int. J. Corros. Scale Inhib. 9, 1237 (2020).
Ş. Erdoğan, Z. S. Safi, S. Kaya, et al., J. Mol. Struct. 1134, 751 (2017).
S. Kaya, C. Kaya, and N. Islam, Comput. Theor. Chem. 1080, 72 (2016).
B. Gómez, N. V. Likhanova, M. A. Domínguez-Aguilar, et al., J. Phys. Chem. B 110, 8928 (2006).
F. Mendez, M. Galván, A. Garritz, et al., J. Mol. Struct.: THEOCHEM 277, 81 (1992).
S. Kumar, D. G. Ladha, P. C. Jha, and N. K. Shah, Int. J. Corros. 2013, 819643 (2013).
R. G. Parr and W. Yang, J. Am. Chem. Soc. 106, 4049 (1984).
R. G. Parr, S. R. Gadre, and L. J. Bartolotti, Proc. Natl. Acad. Sci. U. S. A. 76, 2522 (1979).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Mamand, D., Qadr, H. Density Functional Theory and Computational Simulation of the Molecular Structure on Corrosion of Carbon Steel in Acidic Media of Some Amino Acids. Russ. J. Phys. Chem. 96, 2155–2165 (2022). https://doi.org/10.1134/S0036024422100193
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024422100193