Abstract
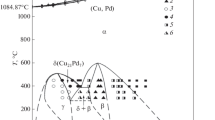
The phase diagram of the nickel–platinum system was constructed with extrapolation of phase equilibria to absolute zero temperature according to the requirements of the third law of thermodynamics. An fcc continuous solid solution crystallizing from the melt undergoes ordering with separation into three intermetallic phases (Kurnakov phases), whose homogeneity regions with decreasing temperature tend to stoichiometric compositions Ni3Pt, NiPt, and NiPt3. With increasing temperature, platinum and nickel occupy some of the crystallographic positions of the second component in the intermetallic phases. In the solid state, in the system, there are two eutectoids with the coordinates (500°C, 36 at % Pt) and (480°C, 64 at % Pt) and three dystectoids with the coordinates (515°C, 31 at % Pt), (645°C, 50 at %Pt), and (510°C, 72 at % Pt).


Similar content being viewed by others
REFERENCES
N. S. Kurnakow and W. A. Nemilov, Z. Anorg. Allg. Chem. 210, 1 (1933).
M. Khansen and K. Anderko, Structures of Double Alloys (Metallurgizdat, Moscow, 1962) [in Russian].
A. Kusmann and H. Nitka, Phys. Z. 39, 373 (1938).
A. Kusmann and H. E. Steinwehr, Z. Met. 40, 263 (1949).
U. Esch and A. Schneider, Z. Electrochem. 50, 268 (1944).
R. S. Oriani and T. S. Jones, Acta Metall. 1, 243 (1953).
G. C. Woolley and B. Bates, J. Less-Common Met. 2, 11 (1960).
M. Greenholz, A. Kidron, and U. Shimony, J. Mater. Sci. 7, 1285 (1972).
A. R. Miedema, J. Less-Common Met. 46, 67 (1976).
G. T. Stevens, M. Hatherly, and J. S. Bowles, J. Mater. Sci. 13, 499 (1978).
C. E. Dahmani, M. C. Cadeville, J. M. Sanchez, and J. L. Moran-Lopez, Phys. Rev. Lett. 55, 1208 (1985).
M. C. Cadeville, C. E. Dahmani, and F. Kern, J. Magn. Magn. Mater. 54–57, 1055 (1986).
P. Nash and M. F. Singleton, Bull. Alloy Phase Diagr. 10, 258 (1989).
X. -G. Lu, B. Sundman, and J. Agren, CALPHAD 33, 450 (2009).
B. Schonfeld, M. Engelke, and A. S. Sologubenko, Philos. Mag. 95, 1080 (2015).
A. A. Popov, A. D. Varygin, P. E. Plyusnin, et al., J. Alloys Compd. 891, 161974 (2021). https://doi.org/10.1016/j.jallcom.2021.161974
A. A. Popov, Cand. Sci. (Chem.) Diss. (Novosibirsk, 2021).
Q. C. Tran, H. An, H. Ha, et al., J. Mater. Chem. A 6, 8259 (2018).
S. Ali, I. Khan, S. A. Khan, et al., J. Electroanal. Chem. 795, 17 (2017).
S. S. A. Razee, J. B. Staunton, F. J. Pinski, et al., J. Appl. Phys. 83, 7097 (1998).
N. S. Kurnakov, S. F. Zemczuzny, M. M. Zasedatelev, J. Inst. Met. London, 15, 305 (1916).
P. P. Fedorov and S. N. Volkov, Russ. J. Inorg. Chem. 61, 772 (2016). https://doi.org/10.1134/S0036023616060061
A. A. Smirnov, Molecular Kinetic Theory of Metals (Nauka, Moscow, 1966) [in Russian].
P. P. Fedorov, Russ. J. Inorg. Chem. 37, 973 (1992).
P. P. Fedorov, Russ. J. Inorg. Chem. 55, 1722 (2010). https://doi.org/10.1134/S0036023610110100
P. P. Fedorov, A. A. Alexandrov, V. V. Voronov, et al., J. Am. Ceram. Soc. 104, 2836 (2021). https://doi.org/10.1111/jace.17666
W. Nernst, The New Heat Theorem (Methuen and Co, London, 1917).
J. P. Abriata and D. E. Laughlin, Prog. Mat. Sci. 49, 367 (2004).
D. E. Laughlin and W. A. Soffa, Acta Mater. 145, 49 (2018). https://doi.org/10.1016/j.actamat.2017.11.037
F. A. Kroger, Chemistry of Imperfect Crystals (North-Holland, 1964).
P. P. Fedorov, Yu. V. Shubin, and E. V. Chernova, Russ. J. Inorg. Chem. 66, 891 (2021). https://doi.org/10.1134/S0036023621050053
R. S. Irani, Contemp. Phys. 13, 559 (1972). https://doi.org/10.1080/00107517208228017
A. Aharony, in Lecture Notes in Physics, Ed. by F. J. W. Hahne (Springer, Berlin, 1983). https://doi.org/10.1007/3-540-12675-9_13
P. Pfeuty and G. Toulouse, Introduction to the Renormalization Group and to Critical Phenomena (John Wiley and Sons, London, 1977).
M. A. Anisimov, E. E. Gorodetskii, and V. M. Zaprudskii, Sov. Phys. Usp. 24, 57 (1981). https://doi.org/10.1070/PU1981v024n01ABEH004612
N. E. Phillips, Ann. Rev. Phys. Chem. 19, 559 (1968).
B. N. Esel’son, V. I. Grigor’ev, V. G. Ivantsov, et al., Solutions of Quantum Liquids He 3 –He 4 (Moscow, Nauka, 1973) [in Russian].
J. R. Goldsmith and H. C. Heard, J. Geol. 69, 45 (1961).
P. P. Fedorov, A. A. Alexandrov, S. V. Kuznetsov, and V. V. Voronov, J. Chem. Thermodyn. 149, 106178 (2020). https://doi.org/10.1016/j.jct.2020.106178
P. P. Fedorov, V. Yu. Proydakova, S. V. Kuznetsov, et al., J. Am. Ceram. Soc. 103, 3390 (2020). https://doi.org/10.1111/jace.16996
U. R. Kattner and B. P. Burton, Phase Diagrams of Binary Iron Alloys (ASM International, Materials Park, OH, 12, 1992).
I. Prigozhin and R. Defei, Chemical Thermodynamics (Nauka, Novosibirsk, 1966) [in Russian].
A. I. Gusev, Nonstoichiometry, Disorder, Short-Range and Long-Range Order in Solids (Fizmatlit, Moscow, 2007) [in Russian].
Funding
This work was supported by the Russian Science Foundation (grant no. 22-13-00167, https://rscf.ru/project/22-13-00167/) and also under a state assignment for basic scientific research for the Nikolaev Institute of Inorganic Chemistry, Siberian Branch, Russian Academy of Sciences, Novosibirsk, Russia, (project no. 121031700315-2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Glyanchenko
Rights and permissions
About this article
Cite this article
Fedorov, P.P., Popov, A.A., Shubin, Y.V. et al. Phase Diagram of the Nickel–Platinum System. Russ. J. Inorg. Chem. 67, 2018–2022 (2022). https://doi.org/10.1134/S0036023622601453
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023622601453