Abstract
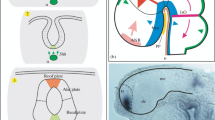
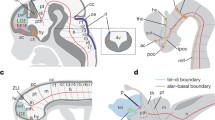
The telencephalon, which provides the highest forms of nervous activity in humans and other animals, is one of the most important innovations of vertebrates. Although this part of the brain has been described in all living vertebrates, its evolutionary origin is still poorly understood. This article discusses one of the possible approaches to studying the expression and functional properties of genes that regulate the early development of the forebrain in cyclostomes (lampreys) as the most archaic representatives of vertebrates. The results of studies of genes such as Anf, FoxG1, and genes of the Noggin family are described.






Similar content being viewed by others
REFERENCES
Bayramov, A.V., Martynova, N.Yu., Eroshkin, F.M., et al., The homeodomain-containing transcription factor X-nkx-5.1 inhibits expression of the homeobox gene Xanf-1 during the Xenopus laevis forebrain development, Mech. Dev., 2004, vol. 121, pp. 1425–1441.
Bayramov, A.V., Eroshkin, F.M., Martynova, N.Y., et al., Novel functions of Noggin proteins: inhibition of Activin/Nodal and Wnt signaling, Development, 2011, vol. 138, pp. 5345–5356.
Bayramov, A.V., Ermakova, G.V., Eroshkin, F.M., et al., The presence of the Anf/Hesx1 homeobox in lampreys indicates that it may play important role in telencephalon emergence, Sci. Rep., 2016, vol. 6, p. 39849.
Bayramov, A.V., Ermakova, G.V., Eroshkin, F.M., et al., Presence of homeobox gene of Anf class in Pacific lamprey Lethenteron camtschaticum confirms the hypothesis about the importance of emergence of Anf genes for the origin of telencephalon in vertebrate evolution, Russ. J. Dev. Biol., 2017, vol. 48, no. 4, pp. 241–251.
Borodulin, A.V., Eroshkin, F.M., Bayramov, A.V., et al., Noggin4 expression during chick embryonic development, Int. J. Dev. Biol., 2013, vol. 56, pp. 403–406.
Botchkarev, V.A., Botchkareva, N.V., Roth, W., et al., Noggin is a mesenchymally derived stimulator of hair-follicle induction, Nat. Cell. Biol., 1999, vol. 1, pp. 158–164.
Brunet, L.J., McMahon, J.A., McMahon, et al., Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton, Science, 1998, vol. 280, pp. 1455–1457.
Cha, S.W. and Heasman, J., Using oocytes for Wnt signaling assays: paracrine assays and wnt-conditioned medium, Methods, 2010, vol. 51, no. 1, pp. 52–55.
Chapman, S.C., Schubert, F.R., Schoenwolf, G.C., et al., Analysis of spatial and temporal gene expression patterns in blastula and gastrula stage chick embryos, Dev. Biol., 2002, vol. 245, no. 1, pp. 187–199.
Dale, L. and Slack, J.M.W., Regional specification within the mesoderm of early embryos of Xenopus laevis, Development, 1987, vol. 100, pp. 279–295.
Danesin, C. and Houart, C., A Fox stops the Wnt: implications for forebrain development and diseases, Curr. Opin. Genet. Dev., 2012, vol. 22, no. 4, pp. 323–330.
Danesin, C., Peres, J.N., Johansson, M., et al., Integration of telencephalic Wnt and hedgehog signaling center activities by Foxg1, Dev. Cell, 2009, vol. 16, no. 4, pp. 576–587.
Ermakova, G.V., Alexandrova, E.M., Kazanskaya, O.V., et al., The homeobox gene, Xanf-1, can control both neural differentiation and patterning in the presumptive anterior neurectoderm of the Xenopus laevis embryo, Development, 1999, vol. 126, pp. 4513–4523.
Ermakova, G.V., Solovieva, E.A., Martynova, N.Y., et al., The homeodomain factor Xanf represses expression of genes in the presumptive rostral forebrain that specify more caudal brain regions, Dev. Biol., 2007, vol. 307, pp. 483–497.
Ermakova, G.V., Kucheryavyy, A.V., Zaraisky, A.G., and Bayramov, A.V., The expression of FoxG1 in the early development of the European river lamprey Lampetra fluviatilis demonstrates significant heterochrony with that in other vertebrates, Gene Expr. Patterns, 2019, vol. 28, no. 34, p. 119073.
Eroshkin, F., Kazanskaya, O., Martynova, N., et al., Characterization of cis-regulatory elements of the homeobox gene Xanf-1, Gene, 2002, vol. 285, pp. 279–286.
Eroshkin, F.M., Ermakova, G.V., Bayramov, A.V., et al., Multiple noggins in vertebrate genome: cloning and expression of noggin2 and noggin4 in Xenopus laevis, Gene Expr. Patterns, 2006, vol. 6, pp. 180–186.
Feinberg, T.E. and Mallatt, J., The evolutionary and genetic origins of consciousness in the Cambrian period over 500 million years ago, Front. Psychol., 2013, vol. 4, p. 667.
Fletcher, R.B., Watson, A.L., and Harland, R.M., Expression of Xenopus tropicalis noggin1 and noggin2 in early development: two noggin genes in a tetrapod, Gene Expr. Patterns, 2004, vol. 5, pp. 225–230.
Furthauer, M., Thisse, B., and Thisse, C., Three different noggin genes antagonize the activity of bone morphogenetic proteins in the zebrafish embryo, Dev. Biol., 1999, vol. 214, pp. 181–196.
Gess, R.W., Coates, M.I., and Rubidge, B.S., A lamprey from the Devonian period of South Africa, Nature, 2006, vol. 443, no. 7114, pp. 981–984.
Green, S.A. and Bronner, M.E., The lamprey: a jawless vertebrate model system for examining origin of the neural crest and other vertebrate traits, Differentiation, 2014, vol. 87, pp. 44–51.
Groppe, J., Greenwald, J., Wiater, E., et al., Structural basis of BMP signalling inhibition by the cystine knot protein noggin, Nature, 2002, vol. 420, pp. 636–642.
Hume, J.B., Adams, C.E., Mable, B., et al., Post-zygotic hybrid viability in sympatric species pairs: a case study from European lampreys, Biol. J. Linn. Soc., 2013, vol. 108, no. 2, pp. 378–383.
Janvier, P., Modern look for ancient lamprey, Nature, 2006, vol. 433, pp. 921–924.
Knecht, A.K. and Harland, R.M., Mechanisms of dorsal-ventral patterning in noggin- induced neural tissue, Development, 1997, vol. 24, pp. 2477–2488.
Koide, T., Hayata, T., and Cho, K.W., Xenopus as a model system to study transcriptional regulatory networks, Proc. Natl. Acad. Sci. U. S. A., 2005, vol. 5, no. 102 (14), pp. 4943–4948.
Kortum, F., Das, S., Flindt, M., et al., The core FOXG1 syndrome phenotype consists of postnatal microcephaly, severe mental retardation, absent language, dyskinesia, and corpus callosum hypogenesis, J. Med. Genet., 2011, vol. 8, no. 6, pp. 396–406.
Kumamoto, T. and Hanashima, C., Evolutionary conservation and conversion of Foxg1 function in brain development, Dev. Growth Differ., 2017, vol. 59, no. 4, pp. 258–269.
Kuraku, S. and Kuratani, S., Time scale for cyclostome evolution inferred with a phylogenetic diagnosis of hagfish and lamprey cDNA sequences, Zool. Sci., 2006, vol. 23, no. 12, pp. 1053–1064.
Kuratani, S., Nobusada, Y., Horigome, N., et al., Embryology of the lamprey and evolution of the vertebrate jaws: insights from molecular and developmental perspectives, Philos. Trans. R. Soc., B, 2001, vol. 356, no. 1414, pp. 1615–1632.
Lamb, T.M., Knecht, A.K., Smith, W.C., et al., Neural induction by secreted polypeptide noggin, Science, 1993, vol. 262, pp. 713–718.
Martynoga, B., Morrison, H., Price, D.J., et al., Foxg1 is required for specification of ventral telencephalon and region-specific regulation of dorsal telencephalic precursor proliferation and apoptosis, Dev. Biol., 2005, vol. 283, no. 1, pp. 113–27.
Martynova, N.Yu., Eroshkin, F.M., Ermakova, G.V., et al., Patterning the forebrain: foxa4a/pintallavis and xvent-2 determine the posterior limit of the xanf-1 expression in the neural plate, Development, 2004, vol. 131, pp. 2329–2338.
McCauley, D.W., Docker, M.F., Whyard, S., et al., Lampreys as diverse model organisms in the genomics era, BioScience, 2015, vol. 65, no. 11, pp. 1046–1056.
McMahon, J.A., Takada, S., Zimmerman, L.B., et al., Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite, Genes Dev., 1998, vol. 12, pp. 1438–1452.
McNamara, K.J., Heterochrony: the evolution of development, Evol. Educ. Outreach, 2012, vol. 5, pp. 203–218.
Meléndez-Ferro, M., Villar-Cheda, B., Abalo, X.M., et al., Early development of the retina and pineal complex in the sea lamprey: comparative immunocytochemical study, J. Comp. Neurol., 2002, vol. 442, no. 3, pp. 250–265.
Moreau, M. and Leclerc, C., The choice between epidermal and neural fate: a matter of calcium, Int. J. Dev. Biol., 2004, vol. 48, nos. 2–3, pp. 75–84.
Murakami, Y., Uchida, K., Rijli, F.M., et al., Evolution of the brain developmental plan: insights from agnathans, Dev. Biol., 2005, vol. 280, pp. 249–259.
Oisi, Y., Ota, K.G., Kuraku, S., et al., Craniofacial development of hagfishes and the evolution of vertebrates, Nature, 2013, vol. 493, no. 7431, pp. 175–180.
Osório, J. and Rétaux, S., The lamprey in evolutionary studies, Dev. Genes Evol., 2008, vol. 218, no. 5, pp. 221–235.
Ota, K.G., Kuraku, S., and Kuratani, S., Hagfish embryology with reference to the evolution of the neural crest, Nature, 2007, vol. 446, pp. 672–675.
Pavlov, D.S., Nazarov, D.Yu., Zvezdin, A.O., Kucherya-vyi, A.V., et al., Downstream migration of early larvae of the European river lamprey Lampetra fluviatilis, Dokl. Biol. Sci., 2014, vol. 459, no. 2, pp. 248–251.
Piavis, G.W., Embryological stages in the sea lamprey and effects of temperature on development, U.S. Fish Wildl. Serv. Biol. Bull., 1961, vol. 182, no. 61, pp. 111–143.
Reese, D.E., Hall, C.E., and Mikawa, T., Negative regulation of midline vascular development by the notochord, Dev. Cell, 2004, vol. 6, pp. 699–708.
Renaud, C.B., Lampreys of the world. An annotated and illustrated catalogue of lamprey species known to date, FAO Spec. Cat. Fish. Purp., 2011, vol. 5, p. 109.
Reyes, R.C., Embryogenesis and ammocoete morphological development of the Pacific lamprey (Entosphenus tridentatus Gairdner, 1836) from the American River, California, Tracy Tech. Bull, 2008, vol. 3, p. 28.
Richardson, M.K. and Wright, G.M., Developmental transformations in a normal series of embryos of the sea lamprey Petromyzon marinus (Linnaeus), J. Morphol., 2003, vol. 257, no. 3, pp. 348–363.
Richardson, M.K., Admiraal, J., and Wright, G.M., Developmental anatomy of lampreys, Biol. Rev. Camb. Philos. Soc., 2010, vol. 85, no. 1, pp. 1–33.
Shimada T, Takai Y, Shinohara K, Yamasaki A, et al., A simplified method to generate serotonergic neurons from mouse embryonic stem and induced pluripotent stem cells, J. Neurochem., 2012, vol. 122, no. 1, pp. 81–93.
Sive, H., Grainger, R.M., and Harland, R.M., Early Development of Xenopus laevis: A Laboratory Manual, CSH Laboratory Press, 2000.
Slack, J.M. and Tannahill, D., Noggin the dorsalizer, Nature, 1993, vol. 361, pp. 498–499.
Smith, A.J., Howell, J.H., and Piavis, G.W., Comparative embryology of five species of lamprey of the Upper Great Lakes, Copeia, 1968, vol. 3, pp. 461–469.
Smith, W.C. and Harland, R.M., Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos, Cell, 1992, vol. 70, pp. 829–840.
Smith, W.C., Knecht, A.K., Wu, M., et al., Secreted noggin protein mimics the Spemann organizer in dorsalizing Xenopus mesoderm, Nature, 1993, vol. 361, pp. 547–549.
Smith, J.J., Kuraku, S., Holt, C., et al., Sequencing of the sea lamprey (Petromyzon marinus) genome provides insights into vertebrate evolution, Nat. Genet., 2013, vol. 45, pp. 415–421.
Square, T., Romasek, M., Jandzik, D., et al., CRISPR/Cas9-mediated mutagenesis in the sea lamprey Petromyzon marinus: a powerful tool for understanding ancestral gene functions in vertebrates, Development, 2015, vol. 142, no. 23, pp. 4180–4187.
Suda, Y., Kurokawa, D., Takeuchi, M., et al., Evolution of Otx paralogue usages in early patterning of the vertebrate head, Dev. Biol., 2009, vol. 325, no. 1, pp. 282–295.
Sugahara, F., Murakami, Y., and Kuratani, S., Gene Expression Analysis of Lamprey Embryo, New York: Springer, 2015.
Sugahara, F., Pascual-Anaya, J., Oisi, Y., et al., Evidence from cyclostomes for complex regionalization of the ancestral vertebrate brain, Nature, 2016, vol. 531, pp. 97–100.
Sugahara, F., Murakami, Y., Pascual-Anaya, J., et al., Reconstructing the ancestral vertebrate brain, Dev. Growth Differ., 2017, vol. 59, no. 4, pp. 163–174.
Suzuki, D.G., Murakami, Y., Escriva, H., et al., A comparative examination of neural circuit and brain patterning between the lamprey and amphioxus reveals the evolutionary origin of the vertebrate visual center, J. Comp. Neurol., 2015, vol. 523, no. 2, pp. 251–261.
Tahara, Y., Normal stages of development in the lamprey, Lampetra reissneri (Dybowski), Zool. Sci., 1988, vol. 5, pp. 109–118.
Tomsa, J.M. and Langeland, J.A., Otx expression during lamprey embryogenesis provides insights into the evolution of the vertebrate head and jaw, Dev. Biol., 1999, vol. 207, no. 1, pp. 26–37.
Tsimbalov, I.A., Kucheryavyi, A.V., and Pavlov, D.S., Results of hybridization between anadromous and resident forms of European river lamprey Lampetra fluviatilis, J. Ichthyol., 2018, vol. 58, no. 1, pp. 122–126.
Warren, S.M., Brunet, L.J., Harland, R.M., et al., The bmp antagonist noggin regulates cranial suture fusion, Nature, 2003, vol. 422, pp. 625–629.
Watanabe, K., Kamiya, D., Nishiyama, A., et al., Direct differentiation of telencephalic precursors from embryonic stem cells, Nat. Neurosci., 2005, vol. 8, pp. 288–296.
Wheeler, G.N. and Brandli, A.W., Simple vertebrate models for chemical genetics and drug discovery screens: lessons from zebrafish and Xenopus, Dev. Dyn., 2009, vol. 238, no. 6, pp. 1287–308.
Xanthos JB, Kofron M, Tao Q et al., The roles of three signaling pathways in the formation and function of the Spemann Organizer // Development. 2002. V. 129(17). P. 4027-43.
Yang, X.U., Si-Wei, Z.H.U., and Qing-Wei, L.I., Lamprey: a model for vertebrate evolutionary research, Dongwuxue Yanjiu, 2016, vol. 37, no. 5, pp. 263–269.
Zaraisky, A.G., Lukyanov, S.A., Vasiliev, O.L., et al., A novel homeobox gene expressed in the anterior neural plate of the Xenopus embryo, Dev. Biol., 1992, vol. 152, pp. 373–382.
Zu, Y., Zhang, X., Ren, J., et al., Biallelic editing of a lamprey genome using the CRISPR/Cas9 system, Sci. Rep., 2016, vol. 6, p. 23496.
Funding
This study was supported by the Russian Foundation for Basic Research (project no. 19-14-50215). Experiments on the study of the FoxG1 and Noggin genes in lampreys were supported by the Russian Foundation for Basic Research (project no. 18-04-00015). Experiments on the analysis of the expression of forehead genes on Xenopus embryos were supported by the Russian Science Foundation (project no. 19-14-00098). Experiments on the functional study of Xenopus forehead genes were supported by the Russian Foundation for Basic Research (project no. 18-29-07014). The study of the Anf gene was supported by the program of the Russian Academy of Sciences “Basic Research for Biomedical Technologies.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by M. Batrukova
Rights and permissions
About this article
Cite this article
Ermakova, G.V., Kucheryavyy, A.V., Eroshkin, F.M. et al. Study of the Early Telencephalon Genes of Cyclostomes as a Way to Restoring the Evolutionary History of This Unique Part of the Central Nervous System of Vertebrates. Paleontol. J. 55, 752–765 (2021). https://doi.org/10.1134/S0031030121070030
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0031030121070030