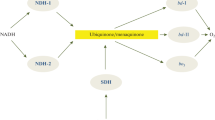
Abstract
An overview of current notions on the mechanism of generation of a transmembrane electric potential difference (Δψ) during the catalytic cycle of a bd-type triheme terminal quinol oxidase is presented in this work. It is suggested that the main contribution to Δψ formation is made by the movement of H+ across the membrane along the intra-protein hydrophilic proton-conducting pathway from the cytoplasm to the active site for oxygen reduction of this bacterial enzyme.





Similar content being viewed by others
Abbreviations
- Δψ:
-
transmembrane electrical potential difference
- Δp :
-
protonmotive force
- τ:
-
time constant, reciprocal of rate constant (t1/e)
- H+/e– :
-
proton/electron stoichiometry which in the case of Escherichia coli respiratory chain means number of protons released into the periplasm per electron used to reduce oxygen to water
- RuBpy:
-
tris(2,2′-bipyridyl)ruthenium(II) chloride
References
Drachev, L. A., Jasaitis, A. A., Kaulen, A. D., Kondrashin, A. A., Liberman, E. A., Nemecek, I. B., Ostroumov, S. A., Semenov, A. Y., and Skulachev, V. P. (1974) Direct measurement of electric current generation by cytochrome oxidase, H+-ATPase and bacteriorhodopsin, Nature, 249, 321-324, https://doi.org/10.1038/249321a0.
Drachev, L. A., Kaulen, A. D., Khitrina, L. V., and Skulachev, V. P. (1981) Fast stages of photoelectric processes in biological membranes. I. Bacteriorhodopsin, Eur. J. Biochem., 117, 461-470, https://doi.org/10.1111/j.1432-1033.1981.tb06361.x.
Dracheva, S. M., Drachev, L. A., Konstantinov, A. A., Semenov, A. Y., Skulachev, V. P., Arutjunjan, A. M., Shulalov, V. A., and Zaberezhnaya, S. M. (1988) Electrogenic steps in the redox reactions catalyzed by photosynthetic reaction-centre complex from Rhodopseudomonas viridis, Eur. J. Biochem., 171, 253-264, https://doi.org/10.1111/j.1432-1033.1988.tb13784.x.
Mulkidjanian, A. Y., Mamedov, M. D., Semenov, A. Y., Shinkarev, V. P., Verkhovsky, M. I., and Drachev, L. A. (1990) Partial reversion of the electrogenic reaction in the ubiquinol: cytochrome c2-oxidoreductase of Rhodobacter sphaeroides chromatophores under neutral and alkaline conditions, FEBS Lett., 277, 127-130, https://doi.org/10.1016/0014-5793(90)80825-4.
Zaslavsky, D., Kaulen, A. D., Smirnova, I. A., Vygodina, T., and Konstantinov, A. A. (1993) Flash-induced membrane potential generation by cytochrome c oxidase, FEBS Lett., 336, 389-393, https://doi.org/10.1016/0014-5793(93)80843-j.
Konstantinov, A. A., Siletsky, S., Mitchell, D., Kaulen, A., and Gennis, R. B. (1997) The roles of the two proton input channels in cytochrome c oxidase from Rhodobacter sphaeroides probed by the effects of site-directed mutations on time-resolved electrogenic intraprotein proton transfer, Proc. Natl. Acad. Sci. USA, 94, 9085-9090, https://doi.org/10.1073/pnas.94.17.9085.
Siletsky, S., Kaulen, A. D., and Konstantinov, A. A. (1999) Resolution of electrogenic steps couples to conversion of cytochrome c oxidase from the peroxy to the ferryl-oxo state, Biochemistry, 38, 4853-4861, https://doi.org/10.1021/bi982614a.
Bogachev, A. V., Bertsova, Y. V., Verkhovskaya, M. L., Mamedov, M. D., and Skulachev, V. P. (2016) Real-time kinetics of electrogenic Na+ transport by rhodopsin from the marine flavobacterium Dokdonia sp. PRO95, Sci. Rep., 6, 21397, https://doi.org/10.1038/srep21397.
Siletsky, S. A., Lukashev, E. P., Mamedov, M. D., Borisov, V. B., Balashov, S. P., Dolgikh, D. A., Rubin, A. B., Kirpichnikov, M. P., and Petrovskaya, L. E. (2021) His57 controls the efficiency of ESR, a light-driven proton pump from Exiguobacterium sibiricum at low and high pH, Biochim. Biophys. Acta Bioenerg., 1862, 148328, https://doi.org/10.1016/j.bbabio.2020.148328.
Verkhovsky, M. I., Morgan, J. E., Verkhovskaya, M., and Wikstrom, M. (1997) Translocation of electrical charge during a single turnover of cytochrome-c oxidase, Biochim. Biophys. Acta, 1318, 6-10, https://doi.org/10.1016/S0005-2728(96)00147-8.
Gibson, Q., and Greenwood, C. (1963) Reactions of cytochrome oxidase with oxygen and carbon monoxide, Biochem. J., 86, 541-554, https://doi.org/10.1042/bj0860541.
Siletsky, S. A., Borisov, V. B., and Mamedov, M. D. (2017) Photosystem II and terminal respiratory oxidases: molecular machines operating in opposite directions, Front. Biosci. (Landmark Ed.), 22, 1379-1426, https://doi.org/10.2741/4550.
Azarkina, N. V., Borisov, V. B., Oleynikov, I. P., Sudakov, R. V., and Vygodina, T. V. (2023) Interaction of terminal oxidases with amphipathic molecules, Int. J. Mol. Sci., 24, 6428, https://doi.org/10.3390/ijms24076428.
Zharova, T. V., Grivennikova, V. G., and Borisov, V. B. (2023) F1•Fo ATP Synthase/ATPase: contemporary view on unidirectional catalysis, Int. J. Mol. Sci., 24, 5417, https://doi.org/10.3390/ijms24065417.
Arutyunyan, A. M., Sakamoto, J., Inadome, M., Kabashima, Y., and Borisov, V. B. (2012) Optical and magneto-optical activity of cytochrome bd from Geobacillus thermodenitrificans, Biochim. Biophys. Acta, 1817, 2087-2094, https://doi.org/10.1016/j.bbabio.2012.06.009.
Borisov, V. B., and Siletsky, S. A. (2019) Features of organization and mechanism of catalysis of two families of terminal oxidases: heme-copper and bd-type, Biochemistry (Moscow), 84, 1390-1402, https://doi.org/10.1134/S0006297919110130.
Murali, R., Gennis, R. B., and Hemp, J. (2021) Evolution of the cytochrome bd oxygen reductase superfamily and the function of CydAA′ in Archaea, ISME J., 15, 3534-3548, https://doi.org/10.1038/s41396-021-01019-4.
Siletsky, S. A., and Borisov, V. B. (2021) Proton pum** and non-pum** terminal respiratory oxidases: Active sites intermediates of these molecular machines and their derivatives, Int. J. Mol. Sci., 22, 10852, https://doi.org/10.3390/ijms221910852.
Forte, E., Borisov, V. B., Vicente, J. B., and Giuffre, A. (2017) Cytochrome bd and gaseous ligands in bacterial physiology, Adv. Microb. Physiol., 71, 171-234, https://doi.org/10.1016/bs.ampbs.2017.05.002.
Borisov, V. B., Gennis, R. B., Hemp, J., and Verkhovsky, M. I. (2011) The cytochrome bd respiratory oxygen reductases, Biochim. Biophys. Acta, 1807, 1398-1413, https://doi.org/10.1016/j.bbabio.2011.06.016.
Borisov, V. B., Siletsky, S. A., Paiardini, A., Hoogewijs, D., Forte, E., Giuffre, A., and Poole, R. K. (2021) Bacterial oxidases of the cytochrome bd family: Redox enzymes of unique structure, function and utility as drug targets, Antioxid. Redox Signal., 34, 1280-1318, https://doi.org/10.1089/ars.2020.8039.
Friedrich, T., Wohlwend, D., and Borisov, V. B. (2022) Recent advances in structural studies of cytochrome bd and its potential application as a drug target, Int. J. Mol. Sci., 23, 3166, https://doi.org/10.3390/ijms23063166.
Borisov, V. B. (1996) Cytochrome bd: structure and properties, Biochemistry (Moscow), 61, 565-574.
Borisov, V. B., and Verkhovsky, M. I. (2015) Oxygen as acceptor, EcoSal Plus, 6, https://doi.org/10.1128/ecosalplus.ESP-0012-2015.
Puustinen, A., Finel, M., Haltia, T., Gennis, R. B., and Wikstrom, M. (1991) Properties of the two terminal oxidases of Escherichia coli, Biochemistry, 30, 3936-3942, https://doi.org/10.1021/bi00230a019.
Borisov, V. B., Murali, R., Verkhovskaya, M. L., Bloch, D. A., Han, H., Gennis, R. B., and Verkhovsky, M. I. (2011) Aerobic respiratory chain of Escherichia coli is not allowed to work in fully uncoupled mode, Proc. Natl. Acad. Sci. USA, 108, 17320-17324, https://doi.org/10.1073/pnas.1108217108.
Forte, E., Borisov, V. B., Konstantinov, A. A., Brunori, M., Giuffre, A., and Sarti, P. (2007) Cytochrome bd, a key oxidase in bacterial survival and tolerance to nitrosative stress, Ital. J. Biochem., 56, 265-269.
Giuffre, A., Borisov, V. B., Mastronicola, D., Sarti, P., and Forte, E. (2012) Cytochrome bd oxidase and nitric oxide: From reaction mechanisms to bacterial physiology, FEBS Lett., 586, 622-629, https://doi.org/10.1016/j.febslet.2011.07.035.
Giuffre, A., Borisov, V. B., Arese, M., Sarti, P., and Forte, E. (2014) Cytochrome bd oxidase and bacterial tolerance to oxidative and nitrosative stress, Biochim. Biophys. Acta, 1837, 1178-1187, https://doi.org/10.1016/j.bbabio.2014.01.016.
Borisov, V. B., Forte, E., Siletsky, S. A., Arese, M., Davletshin, A. I., Sarti, P., and Giuffre, A. (2015) Cytochrome bd protects bacteria against oxidative and nitrosative stress: a potential target for next-generation antimicrobial agents, Biochemistry (Moscow), 80, 565-575, https://doi.org/10.1134/S0006297915050077.
Bader, M., Muse, W., Ballou, D. P., Gassner, C., and Bardwell, J. C. A. (1999) Oxidative protein folding is driven by the electron transport system, Cell, 98, 217-227, https://doi.org/10.1016/S0092-8674(00)81016-8.
Mobius, K., Arias-Cartin, R., Breckau, D., Hannig, A. L., Riedmann, K., Biedendieck, R., Schroder, S., Becher, D., Magalon, A., Moser, J., Jahn, M., and Jahn, D. (2010) Heme biosynthesis is coupled to electron transport chains for energy generation, Proc. Natl. Acad. Sci. USA, 107, 10436-10441, https://doi.org/10.1073/pnas.1000956107.
Seregina, T. A., Lobanov, K. V., Shakulov, R. S., and Mironov, A. S. (2022) Inactivation of terminal oxidase bd-I leads to supersensitivity of E. coli to quinolone and beta-lactam antibiotics, Mol. Biol. (Mosk), 56, 619-627, https://doi.org/10.1134/S0026893322040100.
Borisov, V. B., Forte, E., Siletsky, S. A., Sarti, P., and Giuffre, A. (2015) Cytochrome bd from Escherichia coli catalyzes peroxynitrite decomposition, Biochim. Biophys. Acta, 1847, 182-188, https://doi.org/10.1016/j.bbabio.2014.10.006.
Borisov, V. B., Forte, E., Konstantinov, A. A., Poole, R. K., Sarti, P., and Giuffre, A. (2004) Interaction of the bacterial terminal oxidase cytochrome bd with nitric oxide, FEBS Lett., 576, 201-204, https://doi.org/10.1016/j.febslet.2004.09.013.
Borisov, V. B., Forte, E., Sarti, P., Brunori, M., Konstantinov, A. A., and Giuffre, A. (2006) Nitric oxide reacts with the ferryl-oxo catalytic intermediate of the CuB-lacking cytochrome bd terminal oxidase, FEBS Lett., 580, 4823-4826, https://doi.org/10.1016/j.febslet.2006.07.072.
Borisov, V. B., Forte, E., Sarti, P., Brunori, M., Konstantinov, A. A., and Giuffre, A. (2007) Redox control of fast ligand dissociation from Escherichia coli cytochrome bd, Biochem. Biophys. Res. Commun., 355, 97-102, https://doi.org/10.1016/j.bbrc.2007.01.118.
Mason, M. G., Shepherd, M., Nicholls, P., Dobbin, P. S., Dodsworth, K. S., Poole, R. K., and Cooper, C. E. (2009) Cytochrome bd confers nitric oxide resistance to Escherichia coli, Nat. Chem. Biol., 5, 94-96, https://doi.org/10.1038/nchembio.135.
Borisov, V. B., Forte, E., Giuffre, A., Konstantinov, A., and Sarti, P. (2009) Reaction of nitric oxide with the oxidized di-heme and heme-copper oxygen-reducing centers of terminal oxidases: different reaction pathways and end-products, J. Inorg. Biochem., 103, 1185-1187, https://doi.org/10.1016/j.**orgbio.2009.06.002.
Shepherd, M., Achard, M. E., Idris, A., Totsika, M., Phan, M. D., Peters, K. M., Sarkar, S., Ribeiro, C. A., Holyoake, L. V., Ladakis, D., Ulett, G. C., Sweet, M. J., Poole, R. K., McEwan, A. G., and Schembri, M. A. (2016) The cytochrome bd-I respiratory oxidase augments survival of multidrug-resistant Escherichia coli during infection, Sci. Rep., 6, 35285, https://doi.org/10.1038/srep35285.
Borisov, V. B., and Forte, E. (2022) Bioenergetics and reactive nitrogen species in bacteria, Int. J. Mol. Sci., 23, 7321, https://doi.org/10.3390/ijms23137321.
Forte, E., Siletsky, S. A., and Borisov, V. B. (2021) In Escherichia coli ammonia inhibits cytochrome bo3 but activates cytochrome bd-I, Antioxidants (Basel), 10, 13, https://doi.org/10.3390/antiox10010013.
Forte, E., Borisov, V. B., Falabella, M., Colaco, H. G., Tinajero-Trejo, M., Poole, R. K., Vicente, J. B., Sarti, P., and Giuffre, A. (2016) The terminal oxidase cytochrome bd promotes sulfide-resistant bacterial respiration and growth, Sci. Rep., 6, 23788, https://doi.org/10.1038/srep23788.
Borisov, V. B., and Forte, E. (2021) Terminal oxidase cytochrome bd protects bacteria against hydrogen sulfide toxicity, Biochemistry (Moscow), 86, 22-32, https://doi.org/10.1134/S000629792101003X.
Borisov, V. B., and Forte, E. (2021) Impact of hydrogen sulfide on mitochondrial and bacterial bioenergetics, Int. J. Mol. Sci., 22, 12688, https://doi.org/10.3390/ijms222312688.
Borisov, V., Gennis, R., and Konstantinov, A. A. (1995) Peroxide complex of cytochrome bd: kinetics of generation and stability, Biochem. Mol. Biol. Int., 37, 975-982.
Borisov, V. B., Gennis, R. B., and Konstantinov, A. A. (1995) Interaction of cytochrome bd from Escherichia coli with hydrogen peroxide, Biochemistry (Moscow), 60, 231-239.
Lindqvist, A., Membrillo-Hernandez, J., Poole, R. K., and Cook, G. M. (2000) Roles of respiratory oxidases in protecting Escherichia coli K12 from oxidative stress, Antonie Van Leeuwenhoek, 78, 23-31, https://doi.org/10.1023/a:1002779201379.
Borisov, V. B., Davletshin, A. I., and Konstantinov, A. A. (2010) Peroxidase activity of cytochrome bd from Escherichia coli, Biochemistry (Moscow), 75, 428-436, https://doi.org/10.1134/S000629791004005X.
Borisov, V. B., Forte, E., Davletshin, A., Mastronicola, D., Sarti, P., and Giuffre, A. (2013) Cytochrome bd oxidase from Escherichia coli displays high catalase activity: an additional defense against oxidative stress, FEBS Lett., 587, 2214-2218, https://doi.org/10.1016/j.febslet.2013.05.047.
Forte, E., Borisov, V. B., Davletshin, A., Mastronicola, D., Sarti, P., and Giuffre, A. (2013) Cytochrome bd oxidase and hydrogen peroxide resistance in Mycobacterium tuberculosis, MBio, 4, e01006-01013, https://doi.org/10.1128/mBio.01006-13.
Al-Attar, S., Yu, Y., Pinkse, M., Hoeser, J., Friedrich, T., Bald, D., and de Vries, S. (2016) Cytochrome bd displays significant quinol peroxidase activity, Sci. Rep., 6, 27631, https://doi.org/10.1038/srep27631.
Borisov, V. B., Siletsky, S. A., Nastasi, M. R., and Forte, E. (2021) ROS defense systems and terminal oxidases in bacteria, Antioxidants (Basel), 10, 839, https://doi.org/10.3390/antiox10060839.
Forte, E., Nastasi, M. R., and Borisov, V. B. (2022) Preparations of terminal oxidase cytochrome bd-II isolated from Escherichia coli reveal significant hydrogen peroxide scavenging activity, Biochemistry (Moscow), 87, 720-730, https://doi.org/10.1134/S0006297922080041.
Theßeling, A., Rasmussen, T., Burschel, S., Wohlwend, D., Kagi, J., Muller, R., Bottcher, B., and Friedrich, T. (2019) Homologous bd oxidases share the same architecture but differ in mechanism, Nat. Commun., 10, 5138, https://doi.org/10.1038/s41467-019-13122-4.
Safarian, S., Hahn, A., Mills, D. J., Radloff, M., Eisinger, M. L., Nikolaev, A., Meier-Credo, J., Melin, F., Miyoshi, H., Gennis, R. B., Sakamoto, J., Langer, J. D., Hellwig, P., Kuhlbrandt, W., and Michel, H. (2019) Active site rearrangement and structural divergence in prokaryotic respiratory oxidases, Science, 366, 100-104, https://doi.org/10.1126/science.aay0967.
Grauel, A., Kagi, J., Rasmussen, T., Makarchuk, I., Oppermann, S., Moumbock, A. F. A., Wohlwend, D., Muller, R., Melin, F., Gunther, S., Hellwig, P., Bottcher, B., and Friedrich, T. (2021) Structure of Escherichia coli cytochrome bd-II type oxidase with bound aurachin D, Nat. Commun., 12, 6498, https://doi.org/10.1038/s41467-021-26835-2.
Grund, T. N., Radloff, M., Wu, D., Goojani, H. G., Witte, L. F., Josting, W., Buschmann, S., Muller, H., Elamri, I., Welsch, S., Schwalbe, H., Michel, H., Bald, D., and Safarian, S. (2021) Mechanistic and structural diversity between cytochrome bd isoforms of Escherichia coli, Proc. Natl. Acad. Sci. USA, 118, e2114013118, https://doi.org/10.1073/pnas.2114013118.
Yang, K., Borisov, V. B., Konstantinov, A. A., and Gennis, R. B. (2008) The fully oxidized form of the cytochrome bd quinol oxidase from E. coli does not participate in the catalytic cycle: direct evidence from rapid kinetics studies, FEBS Lett., 582, 3705-3709, https://doi.org/10.1016/j.febslet.2008.09.038.
Borisov, V. B., Forte, E., Sarti, P., and Giuffre, A. (2011) Catalytic intermediates of cytochrome bd terminal oxidase at steady-state: Ferryl and oxy-ferrous species dominate, Biochim. Biophys. Acta, 1807, 503-509, https://doi.org/10.1016/j.bbabio.2011.02.007.
Borisov, V. B., Smirnova, I. A., Krasnosel’skaya, I. A., and Konstantinov, A. A. (1994) Oxygenated cytochrome bd from Escherichia coli can be converted into the oxidized form by lipophilic electron acceptors, Biochemistry (Moscow), 59, 437-443.
D'mello, R., Hill, S., and Poole, R. K. (1996) The cytochrome bd quinol oxidase in Escherichia coli has an extremely high oxygen affinity and two-oxygen-binding haems: implicaitons for regulation of activity in vivo by oxygen inihibition, Microbiology, 142, 755-763, https://doi.org/10.1099/00221287-142-4-755.
Belevich, I., Borisov, V. B., Konstantinov, A. A., and Verkhovsky, M. I. (2005) Oxygenated complex of cytochrome bd from Escherichia coli: stability and photolability, FEBS Lett., 579, 4567-4570, https://doi.org/10.1016/j.febslet.2005.07.011.
Belevich, I., Borisov, V. B., Bloch, D. A., Konstantinov, A. A., and Verkhovsky, M. I. (2007) Cytochrome bd from Azotobacter vinelandii: evidence for high-affinity oxygen binding, Biochemistry, 46, 11177-11184, https://doi.org/10.1021/bi700862u.
Siletsky, S. A., Zaspa, A. A., Poole, R. K., and Borisov, V. B. (2014) Microsecond time-resolved absorption spectroscopy used to study CO compounds of cytochrome bd from Escherichia coli, PLoS One, 9, e95617, https://doi.org/10.1371/journal.pone.0095617.
Siletsky, S. A., Rappaport, F., Poole, R. K., and Borisov, V. B. (2016) Evidence for fast electron transfer between the high-spin haems in cytochrome bd-I from Escherichia coli, PLoS One, 11, e0155186, https://doi.org/10.1371/journal.pone.0155186.
Hill, J. J., Alben, J. O., and Gennis, R. B. (1993) Spectroscopic evidence for a heme-heme binuclear center in the cytochrome bd ubiquinol oxidase from Escherichia coli, Proc. Natl. Acad. Sci. USA, 90, 5863-5867, https://doi.org/10.1073/pnas.90.12.5863.
Muntyan, M. S., Bloch, D. A., Drachev, L. A., and Skulachev, V. P. (1993) Kinetics of CO binding to putative Na+-motive oxidases of the o-type from Bacillus FTU and of the d-type from Escherichia coli, FEBS Lett., 327, 347-350, https://doi.org/10.1016/0014-5793(93)81018-u.
Tsubaki, M., Hori, H., Mogi, T., and Anraku, Y. (1995) Cyanide-binding site of bd-type ubiquinol oxidase from Escherichia coli, J. Biol. Chem., 270, 28565-28569, https://doi.org/10.1074/jbc.270.48.28565.
Borisov, V., Arutyunyan, A. M., Osborne, J. P., Gennis, R. B., and Konstantinov, A. A. (1999) Magnetic circular dichroism used to examine the interaction of Escherichia coli cytochrome bd with ligands, Biochemistry, 38, 740-750, https://doi.org/10.1021/bi981908t.
Vos, M. H., Borisov, V. B., Liebl, U., Martin, J. L., and Konstantinov, A. A. (2000) Femtosecond resolution of ligand-heme interactions in the high-affinity quinol oxidase bd: A di-heme active site? Proc. Natl. Acad. Sci. USA, 97, 1554-1559, https://doi.org/10.1073/pnas.030528197.
Borisov, V. B., Sedelnikova, S. E., Poole, R. K., and Konstantinov, A. A. (2001) Interaction of cytochrome bd with carbon monoxide at low and room temperatures: evidence that only a small fraction of heme b595 reacts with CO, J. Biol. Chem., 276, 22095-22099, https://doi.org/10.1074/jbc.M011542200.
Borisov, V. B., Liebl, U., Rappaport, F., Martin, J. L., Zhang, J., Gennis, R. B., Konstantinov, A. A., and Vos, M. H. (2002) Interactions between heme d and heme b595 in quinol oxidase bd from Escherichia coli: a photoselection study using femtosecond spectroscopy, Biochemistry, 41, 1654-1662, https://doi.org/10.1021/bi0158019.
Arutyunyan, A. M., Borisov, V. B., Novoderezhkin, V. I., Ghaim, J., Zhang, J., Gennis, R. B., and Konstantinov, A. A. (2008) Strong excitonic interactions in the oxygen-reducing site of bd-type oxidase: the Fe-to-Fe distance between hemes d and b595 is 10 Å, Biochemistry, 47, 1752-1759, https://doi.org/10.1021/bi701884g.
Borisov, V. B. (2008) Interaction of bd-type quinol oxidase from Escherichia coli and carbon monoxide: heme d binds CO with high affinity, Biochemistry (Moscow), 73, 14-22, https://doi.org/10.1134/S0006297908010021.
Bloch, D. A., Borisov, V. B., Mogi, T., and Verkhovsky, M. I. (2009) Heme/heme redox interaction and resolution of individual optical absorption spectra of the hemes in cytochrome bd from Escherichia coli, Biochim. Biophys. Acta, 1787, 1246-1253, https://doi.org/10.1016/j.bbabio.2009.05.003.
Rappaport, F., Zhang, J., Vos, M. H., Gennis, R. B., and Borisov, V. B. (2010) Heme-heme and heme-ligand interactions in the di-heme oxygen-reducing site of cytochrome bd from Escherichia coli revealed by nanosecond absorption spectroscopy, Biochim. Biophys. Acta, 1797, 1657-1664, https://doi.org/10.1016/j.bbabio.2010.05.010.
Borisov, V. B., and Verkhovsky, M. I. (2013) Accommodation of CO in the di-heme active site of cytochrome bd terminal oxidase from Escherichia coli, J. Inorg. Biochem., 118, 65-67, https://doi.org/10.1016/j.**orgbio.2012.09.016.
Siletsky, S. A., Dyuba, A. V., Elkina, D. A., Monakhova, M. V., and Borisov, V. B. (2017) Spectral-kinetic analysis of recombination reaction of heme centers of bd-type quinol oxidase from Escherichia coli with carbon monoxide, Biochemistry (Moscow), 82, 1354-1366, https://doi.org/10.1134/S000629791711013X.
Jasaitis, A., Borisov, V. B., Belevich, N. P., Morgan, J. E., Konstantinov, A. A., and Verkhovsky, M. I. (2000) Electrogenic reactions of cytochrome bd, Biochemistry, 39, 13800-13809, https://doi.org/10.1021/bi001165n.
Belevich, I., Borisov, V. B., Zhang, J., Yang, K., Konstantinov, A. A., Gennis, R. B., and Verkhovsky, M. I. (2005) Time-resolved electrometric and optical studies on cytochrome bd suggest a mechanism of electron-proton coupling in the di-heme active site, Proc. Natl. Acad. Sci. USA, 102, 3657-3662, https://doi.org/10.1073/pnas.0405683102.
Belevich, I., Borisov, V. B., and Verkhovsky, M. I. (2007) Discovery of the true peroxy intermediate in the catalytic cycle of terminal oxidases by real-time measurement, J. Biol. Chem., 282, 28514-28519, https://doi.org/10.1074/jbc.M705562200.
Borisov, V. B., Belevich, I., Bloch, D. A., Mogi, T., and Verkhovsky, M. I. (2008) Glutamate 107 in subunit I of cytochrome bd from Escherichia coli is part of a transmembrane intraprotein pathway conducting protons from the cytoplasm to the heme b595/heme d active site, Biochemistry, 47, 7907-7914, https://doi.org/10.1021/bi800435a.
Paulus, A., Rossius, S. G., Dijk, M., and de Vries, S. (2012) Oxoferryl-porphyrin radical catalytic intermediate in cytochrome bd oxidases protects cells from formation of reactive oxygen species, J. Biol. Chem., 287, 8830-8838, https://doi.org/10.1074/jbc.M111.333542.
Bekker, M., de Vries, S., Ter Beek, A., Hellingwerf, K. J., and de Mattos, M. J. (2009) Respiration of Escherichia coli can be fully uncoupled via the nonelectrogenic terminal cytochrome bd-II oxidase, J. Bacteriol., 191, 5510-5517, https://doi.org/10.1128/JB.00562-09.
Shepherd, M., Sanguinetti, G., Cook, G. M., and Poole, R. K. (2010) Compensations for diminished terminal oxidase activity in Escherichia coli: cytochrome bd-II-mediated respiration and glutamate metabolism, J. Biol. Chem., 285, 18464-18472, https://doi.org/10.1074/jbc.M110.118448.
Acknowledgments
The author would like to express his deepest gratitude to M. I. Verkhovsky (passed away), I. N. Belevich, N. P. Belevich, D. A. Bloch (passed away), and A. Jasaitis for wonderful time spent measuring electrogenic activity of the enigmatic cytochrome bd.
Funding
This work was financially supported by the Russian Science Foundation (project no. 22-24-00045, https://rscf.ru/en/project/22-24-00045/).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares no conflict of interest in financial or any other sphere. This article does not contain any studies involving animals or human participants performed by author.
Rights and permissions
About this article
Cite this article
Borisov, V.B. Generation of Membrane Potential by Cytochrome bd. Biochemistry Moscow 88, 1504–1512 (2023). https://doi.org/10.1134/S0006297923100073
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297923100073