Abstract
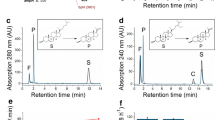
Mycolicibacterium smegmatis mc2155 has been genetically modified to be used as a platform for the expression of heterologous cytochrome P450 monooxygenases by introducing deletions in the kshB and kstD genes that encode key stages of the steroid nucleus enzymatic degradation. Three sets of genetic constructs have been created for heterologous expression of the genes encoding P450 cytochromes CYP106A1 from Bacillus megaterium DSM319 and CYP106A2 from B. megaterium ATCC13368 in M. smegmatis mc2155 (ΔkshBΔkstD) cells. The recombinant plasmids contained monocistronic expression cassettes of cytochrome genes (pNS31 and pNS32), tricistronic cassettes of cytochrome genes together with cDNA copies of adrenodoxin and adrenodoxin reductase genes of the bovine adrenal cortex (pNS33 and pNS34), or monocistronic gene cassettes of chimeric cytochromes fused with the DNA sequence encoding the CYP116B2 reductase domain from the soil bacterium Rhodococcus sp. NCIMB 9784 (pNS35 and pNS36). The recombinant strains of mycolicibacteria were shown to selectively monohydroxylate androstenedione (AD) under growth conditions. The product was identified as 15β-hydroxyandrostenedione (15β-OH-AD) by mass spectrometry and 1H and 13C NMR spectroscopy. The maximum level of 15β-OH-AD production (17.3 ± 1.5 mg/L) was observed when using the recombinant M. smegmatis mc2155 (ΔkshBΔkstD) (pNS32) strain, which expresses a single cyp106A2 gene from B. megaterium ATCC13368. Host proteins of M. smegmatis mc2155 were shown to be capable of supplying electrons to heterologous cytochromes to support their hydroxylation activity. The results we obtained are of a priority character, expand the understanding of the hydroxylation of steroid compounds by bacterial cytochromes CYP106A1/A2, and are important for the creation of microbial strains for producing valuable hydroxysteroids.







Similar content being viewed by others
REFERENCES
Fegan, K.S., Rae, M.T., Critchley, H.O., et al., Antiinflammatory steroid signalling in the human peritoneum, J. Endocrinol., 2008, vol. 196, no. 2, pp. 369–376. https://doi.org/10.1677/joe-07-0419
Ali Shah, S.A., Sultan, S., and Adnan, H.S., A whole-cell biocatalysis application of steroidal drugs, Orient. J. Chem., 2013, vol. 29, no. 2, pp. 389–403. https://doi.org/10.13005/ojc/290201
Milecka-Tronina, N., Kolek, T., Swizdor, A., et al., Hydroxylation of DHEA and its analogues by Absidia coerulea AM93. Can an inducible microbial hydroxylase catalyze 7α- and 7β-hydroxylation of 5-ene and 5α-dihydro C19-steroids?, Bioorg. Med. Chem., 2014, vol. 22, no. 2, pp. 883–891. https://doi.org/10.1016/j.bmc.2013.11.050
Wojtal, K., Trojnar, M.K., and Czuczwar, S.J., Endogenous neuroprotective factors: neurosteroids, Pharmacol. Rep., 2006, vol. 58, no. 3, pp. 335–340.
Reeder, A.Y. and Joannou, G.E., 15β-Hydroxysteroids (Part I). Steroids of the human perinatal period: the synthesis of 3β,15β,17α-trihydroxy-5-pregnen-20-one, Steroids, 1996, vol. 61, no. 2, pp. 74–81. https://doi.org/10.1016/0039-128x(95)00193-t
Bernhardt, R., Cytochromes P450 as versatile biocatalysts, J. Biotechnol., 2006, vol. 124, no. 1, pp. 128–145. https://doi.org/10.1016/j.jbiotec.2006.01.026
Kiss, F.M., Lundemo, M.T., Zapp, J., et al., Process development for the production of 15β-hydroxycyproterone acetate using Bacillus megaterium expressing CYP106A2 as whole-cell biocatalyst, Microb. Cell Fact., 2015, vol. 14, no. 1, p. 28. https://doi.org/10.1186/s12934-015-0210-z
Kiss, F.M., Schmitz, D., Zapp, J., et al., Comparison of CYP106A1 and CYP106A2 from Bacillus megaterium—identifcation of a novel 11-oxidase activity, Appl. Microbiol. Biotechnol., 2015, vol. 99, no. 20, pp. 8495–8514. https://doi.org/10.1007/s00253-015-6563-8
Girhard, M., Klaus, T., Khatri, Y., et al., Characterization of the versatile monooxygenase CYP109B1 from Bacillus subtilis, Appl. Microbiol. Biotechnol., 2010, vol. 87, no. 2, pp. 595–607. https://doi.org/10.1007/s00253-010-2472-z
Jóźwik, I.K., Kiss, F.M., Gricman, Ł., et al., Structural basis of steroid binding and oxidation by the cytochrome P450 CYP109E1 from Bacillus megaterium. FEBS J., 2016, vol. 283, no. 22, pp. 4128–4148. https://doi.org/10.1111/febs.13911
Bracco, P., Janssen, D.B., and Schallmey, A., Selective steroid oxyfunctionalisation by CYP154C5, a bacterial cytochrome P450, Microb. Cell Fact., 2013, vol. 12, no. 1, p. 95. https://doi.org/10.1186/1475-2859-12-95
Litzenburger, M. and Bernhardt, R., CYP260B1 acts as 9α-hydroxylase for 11-deoxycorticosterone, Steroids, 2017, vol. 127, pp. 40–45. https://doi.org/10.1016/j.steroids.2017.08.006
Makino, T., Katsuyama, Y., Otomatsu, T., et al., Regio- and stereospecifc hydroxylation of various steroids at the 16α position of the D ring by the Streptomyces griseus cytochrome P450 CYP154C3, Appl. Environ. Microbiol., 2014, vol. 80, no. 4, pp. 1371–1379. https://doi.org/10.1128/AEM.03504-13
Dangi, B., Kim, K.H., Kang, S.H., et al., Tracking down a novel steroid hydroxylating promiscuous cytochrome P450, CYP154C8 from Streptomyces sp. W2233-SM, ChemBioChem, 2018, vol. 19, no. 10, pp. 1066–1077. https://doi.org/10.1002/cbic.201800018
Agematu, H., Matsumoto, N., Fujii, Y., et al., Hydroxylation of testosterone by bacterial cytochromes P450 using the Escherichia coli expression system, Biosci. Biotech. Biochem., 2006, vol. 70, no. 1, pp. 307–311. https://doi.org/10.1271/bbb.70.307
Zhang, X., Peng, Y., Zhao, J., et al., Bacterial cytochrome P450-catalyzed regio- and stereoselective steroid hydroxylation enabled by directed evolution and rational design, Bioresour. Bioprocess., 2020, vol. 7, p. 2. https://doi.org/10.1186/s40643-019-0290-4
Kim, K.H., Lee, C.W., Dangi, B., et al., Crystal structure and functional characterization of a cytochrome p450 (BaCYP106A2) from Bacillus sp. PAMC 23377, J. Microbiol. Biotechnol., 2017, vol. 27, no. 8, pp. 1472–1482. https://doi.org/10.4014/jmb.1706.06013
Schmitz, D., Zapp, J., and Bernhardt, R., Steroid conversion with CYP106A2 – production of pharmaceutically interesting DHEA metabolites, Microb. Cell. Fact., 2014, vol. 13, p. 81. https://doi.org/10.1186/1475-2859-13-81
Schmitz, D., Janocha, S., Kiss, F.M., et al., CYP106A2—a versatile biocatalyst with high potential for biotechnological production of selectively hydroxylated steroid and terpenoid compounds, Biochim. Biophys. Acta, 2018, vol. 1866, no. 1, pp. 11–22. https://doi.org/10.1016/j.bbapap.2017.07.011
Sagadin, T., Riehm, J.L., Milhim, M., et al., Binding modes of CYP106A2 redox partners determine differences in progesterone hydroxylation product patterns, Commun. Biol., 2018, vol. 1, p. 99. https://doi.org/10.1038/s42003-018-0104-9
Snapper, S.B., Melton, R.E., Mustafa, S., et al., Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis, Mol. Microbiol., 1990, vol. 4, no. 11, pp. 1911–1919. https://doi.org/10.1111/j.1365-2958.1990.tb02040.x
Strizhov, N., Fokina, V., Sukhodolskaya, G., et al., Progesterone biosynthesis by combined action of adrenal steroidogenic and mycobacterial enzymes in fast growing mycobacteria, New Biotechnol., 2014, vol. 31S, p. S67. https://doi.org/10.1016/j.nbt.2014.05.1766
Felpeto-Santero, C., Galan, B., and Garcia, J.L., Production of 11α-hydroxysteroids from sterols in a single fermentation step by Mycolicibacterium smegmatis, Microbiol. Biotechnol., 2021, vol. 14, no. 6, pp. 2514‒2524. https://doi.org/10.1111/1751-7915.13735
Loraine, J.K. and Smith, M.C.M., Genetic techniques for manipulation of the phytosterol biotransformation strain Mycobacterium neoaurum NRRL B-3805, Methods Mol. Biol., 2017, vol. 1645, pp. 93‒108. https://doi.org/10.1007/978-1-4939-7183-1_7
Daugelat, S., Kowall, J., Mattow, J., et al., The RD1 proteins of Mycobacterium tuberculosis: expression in Mycobacterium smegmatis and biochemical characterization, Microbes Infect., 2003, vol. 5, no. 12, pp. 1082–1095. https://doi.org/10.1016/s1286-4579(03)00205-3
Poulsen, C., Holton, S., Geerlof, A., et al., Stoichiometric protein complex formation and over expression using the prokaryotic native operon structure, FEBS Lett., 2010, vol. 584, pp. 669–674. https://doi.org/10.1016/j.febslet.2009.12.057
Strizhov, N., Karpov, M., Sukhodolskaya, G., et al., Development of mycobacterial strains producing testosterone, Izv. Nats. Akad. Nauk Belarusi, Ser. Khim. Nauk, 2016, vol. 3, pp. 57‒58. https://vestichem.belnauka.by/jour/issue/viewIssue/11/2.
Karpov, M.V., Strizhov, N.I., Novikova, L.A., et al., Reconstruction of the cholesterol hydroxylase/lyase enzyme system of the bovine adrenal cortex in rapidly growing mycobacteria, Sbornik tezisov 19-oi Mezhdunarodnoi Pushchinskoi shkoly-konferentsii molodykh uchenykh “Biologiya—nauka 21 veka” (19th International Pushchino School-Conference for Young Scientists “Biology Is a Science of the 21st Century,” Abstracts of Papers), Pushch. Nauchn. Tsentr Ross. Akad. Nauk, Pushchino, 2015, vol. 237–238. https://www.spsl.nsc.ru/FullText/konfe/ITOG-2015.pdf.
Sabbadin, F., Hyde, R., Robin, A., et al., LICRED: a versatile drop-in vector for rapid generation of redoxself-sufficient cytochrome P450s, ChemBioChem, 2010, vol. 11, no. 7, pp. 987–994. https://doi.org/10.1007/978-1-62703-321-3_20
Kollerov, V.V., Lobastova, T.G., Monti, D., et al., Deoxycholic acid transformations catalyzed by selected filamentous fungi, Steroids, 2016, vol. 107, pp. 20–29. https://doi.org/10.1016/j.steroids.2015.12.015
Li, S., Du, L., and Bernhardt, R., Redox partners: function modulators of bacterial P450 enzymes, Trends Microbiol., 2020, vol. 28, no. 6, pp. 445–454. https://doi.org/10.1016/j.tim.2020.02.012
Lisurek, M., Simgen, B., Antes, I., and Bernhardt, R., Theoretical and experimental evaluation of a CYP106A2 low homology model and production of mutants with changed activity and selectivity of hydroxylation, ChemBioChem, 2008, vol. 9, no. 9, pp. 1439–1449. https://doi.org/10.1002/cbic.200700670
Funding
This work was supported by the Russian Science Foundation (project no. 21-64-00024).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
This article does not contain any studies involving animals performed by any of the authors.
This article does not contain any studies involving human participants performed by any of the authors outside the scope of their normal professional activities.
Additional information
Translated by I. Gordon
Abbreviations: AD, androst-4-ene-3,17-dione; ADD, androsta-1,4-diene-3,17-dione; AdR, adrenodoxin reductase; Adx, adrenodoxin; HPLC, high-performance liquid chromatography; MCD, methyl-β-cyclodextrin; MS, mass spectrometry; NMR, nuclear magnetic resonance; OD600, optical density at a wavelength of 600 nm; RBS, ribosome-binding site(s); TLC, thin-layer chromatography; 15β-OH-AD, androst-4-en-15β-ol-3,17-dione.
Rights and permissions
About this article
Cite this article
Karpov, M.V., Nikolaeva, V.M., Fokina, V.V. et al. Creation and Functional Analysis of Mycolicibacterium smegmatis Recombinant Strains Carrying the Bacillary Cytochromes CYP106A1 and CYP106A2 Genes. Appl Biochem Microbiol 58, 947–957 (2022). https://doi.org/10.1134/S0003683822090058
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683822090058