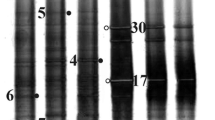
Abstract
The bacterial composition of permafrost samples taken during drilling of frozen marine sediments in the area of Barentsburg coal mine on the east coast of Grønfjord Bay of Western Spitsbergen has been studied. The study was based on the analysis of the V4 region of the 16S rRNA gene, carried out using next generation sequencing, as well as using classical microbiological methods (direct luminescence microscopy and aerobic cultivation).The total cell number in permafrost samples ranges from 6.73 ± 0.73 × 106 to 3.37 ± 0.19 107 cells per g. The number of cultivable aerobic bacteria in frozen samples on 1/5 TSA and R2A media ranges from 0 to 6.20 ± 0.45 × 104 CFU/g. Isolates of aerobic bacteria were identified by 16S rRNA gene analysis as representatives of the genera Arthrobacter, Pseudarthrobacter, Psychrobacter, and Rhodoferax. The dominant phyla of the domain Bacteria were Actinobacteria, Proteobacteria, Chloroflexi, Nitrospirae and Firmicutes. As a result of phylogenetic analysis of the dominant operational taxonomic units, representatives of methane oxidizing, sulfate reducing bacteria, as well as heterotrophic bacteria involved in the transformation of organic matter were found.







Similar content being viewed by others
REFERENCES
Aissa, F.B., Postec, A., Erauso, G., Payri, C., Pelletier, B., Hamdi, M., Ollivier, B., and Fardeau, M.L., Vallitalea pronyensis sp. nov., isolated from a marine alkaline hydrothermal chimney, Int. J. Syst. Evol. Microbiol., 2014, vol. 64, no. 4, pp. 1160–1165.
Albuquerque, L., França, L., Rainey, F.A., Schumann, P., Nobre, M.F., and da Costa, M.S., Gaiella occulta gen. nov., sp. nov., a novel representative of a deep branching phylogenetic lineage within the class Actinobacteria and proposal of Gaiellaceae fam. nov. and Gaiellales ord. nov., Syst. Appl. Microbiol., 2011, vol. 34, no. 8, pp. 595–599.
Alcántara-Hernández, R.J., Centeno, C.M., Ponce-Mendoza, A., Batista, S., Merino-Ibarra, M., Campo, J., and Falcón, L.I., Characterization and comparison of potential denitrifiers in microbial mats from King George Island, Maritime Antarctica, Polar Virol., 2014, vol. 37, no. 3, pp. 403–416.
Alekseev, I.I. and Abakumov, E.V., Taxonomic and environmental soil diversity of marine terraces of Gronfjord (West Spitsbergen Island), Samar. Luka: Probl. Reg. Global. Ekol., 2016, vol. 25, no. 4, pp. 256–161.
Alperin, M.J. and Reeburgh, W.S., Inhibition experiments on anaerobic methane oxidation, App. Environ. Microbiol., 1985, vol. 50, no. 4, pp. 940–945.
Bajerski, F. and Wagner, D., Bacterial succession in Antarctic soils of two glacier forefields on Larsemann Hills, East Antarctica, FEMS Microbiol. Ecol., 2013, vol. 85, no. 1, pp. 128–142.
Baltrus, D.A., Yourstone, S., Lind, A., Guilbaud, C., Sands, D.C., Jones, C.D., Morris, C.E., and Dangl, J.L., Draft genome sequences of a phylogenetically diverse suite of Pseudomonas syringae strains from multiple source populations, Genome Announce., 2014, vol. 2, no. 1, pp. 1–2. https://mra.asm.org/content/ga/2/1/ e01195-13.full.pdf.
Bolyen, E., Rideout, J.R., Dillon, M.R., Bokulich, N.A., Abnet, C.C., Al-Ghalith, G.A., Alexander, H., Alm, E.J., Arumugam, M., Asnicar, F., Bai, Y., Bisanz, J.E., Bittinger, K., Brejnrod, A., Brislawn, C.J., et al., Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2, Nat. Biotechnol., 2019, vol. 37, no. 8, pp. 852–857. Erratum: Nat. Biotechnol., 2019, vol. 37, no. 9, p. 1091.
Borrel, G., Joblin, K., Guedon, A., Colombet, J., Tardy, V., Lehours, A.C., and Fonty, G., Methanobacterium lacus sp. nov., isolated from the profundal sediment of a freshwater meromictic lake, Int. J. Syst. Evol. Microbiol., 2012, vol. 62, no. 7, pp. 1625–1629.
Bowman, J.P. and McCuaig, R.D., Biodiversity, community structural shifts, and biogeography of prokaryotes within Antarctic continental shelf sediment, Appl. Environ. Microbiol., 2003, vol. 69, no. 5, pp. 2463–2483.
Bueno de Mesquita, C.P.B., Schmidt, S.K., and Suding, K.N., Litter-driven feedbacks influence plant colonization of a high elevation early successional ecosystem, Plant Soil, 2019, vol. 444, no. 1, pp. 71–85.
Cabrera-Ospino, M.C., Phylogeny and diversity of genes for poorly characterized type of arsenite oxidase involved in anaerobic arsenic oxidation, Ph.D. Thesis, Japan, 2018.
Carr, S.A., Orcutt, B.N., Mandernack, K.W., and Spear, J.R., Abundant Atribacteria in deep marine sediment from the Adélie Basin, Antarctica, Front. Microbiol., 2015, vol. 6, id 872.
Chao, A., Nonparametric estimation of the number of classes in a population, Scand. J. Stat, 1984, vol. 11, pp. 265–270.
Chen, M.-Y., Wu, Sh.-H., Lin, G.-H., Lu, Ch.-P., Lin, Y.-T., Chang, W.-Ch., and Tsay, S.-S., Rubrobacter taiwanensis sp. nov., a novel thermophilic, radiation-resistant species isolated from hot springs, Int. J. Syst. Evol. Microbiol., 2004, vol. 54, no. 5, pp. 1849–1855.
Chernov, R.A. and Murav’ev, A.Ya., Contemporary changes in the area of glaciers in the western part of the Nordenskjold Land (Svalbard), Led Sneg, 2018, vol. 58, no. 4, pp. 462–472.
Coolen, M.J. and Orsi, W.D., The transcriptional response of microbial communities in thawing Alaskan permafrost soils, Front. Microbiol., 2015, vol. 6, id 197.
Coolen, M.J., Talbot, H.M., Abbas, B.A., Ward, C., Schouten, S., Volkman, J.K., and Damste, J.S., Sources for sedimentary bacteriohopanepolyols as revealed by 16S rDNA stratigraphy, Environ. Microbiol., 2008, vol. 10, no. 7, pp. 1783–1803.
Costello, E.K. and Schmidt, S.K., Microbial diversity in alpine tundra wet meadow soil: Novel Chloroflexi from a cold, water-saturated environment, Environ. Microbiol., 2006, vol. 8, no. 8, pp. 1471–1486.
Cravo-Laureau, C., Matheron, R., Joulian, C., Cayol, J.L., and Hirschler-Rea, A., Desulfatibacillum alkenivorans sp. nov., a novel n-alkene-degrading, sulfate-reducing bacterium, and emended description of the genus desulfatibacillum, Int. J. Syst. Evol. Microbiol., 2004, vol. 54, no. 5, pp. 1639–1642.
Demidov, N.E., Baranskaya, A.V., Durdenko, E.V., Zanina, O.G., Karaevskaya, E.S., Pushina, Z.V., Rivkina, E.M., Spirina, E.V., and Spenser, R., Biogeochemistry of frozen waters of the Arctic coast of the Gydan Peninsula, Probl. Arkt. Antarkt., 2016a, vol. 1, no. 3, pp. 34–49.
Demidov, N.E., Karaevskaya, E.S., Verkulich, S.R., Nikulina, A.L., and Savatyugin, L.M. First results of permafrost monitoring in the cryospheric polygon of the Russian Scientific Center on Spitsbergen (RSCS), Probl. Arkt. Antarkt., 2016b, vol. 1, no. 4, pp. 67–79.
Demidov, N., Wetterich, S., Verkulich, S., Ekaykin, A., Meyer, H., Anisimov, M., Schirrmeister, L., Demidov, V., and Hodson, A.J., Geochemical signatures of **o ice and its origin in Grøndalen, West Spitsbergen, Cryosphere, 2019, vol. 13, pp. 3155–3169.
Demidov, N.E., Borisik, A.L., Verkulich, S.R., Wetterich, S., Gunar, A.Yu., Demidov, V.E., Zheltenkova, N.V., Koshurnikov, A.V., Miloslavskii, M.Yu., Nikulina, A.L., Novikov, A.L., Savatyugin, L.M., Sirotkin, A.N., Terekhov, A.V., Ugryumov, Yu.V., et al., Geocryological and hydrogeological conditions of the western part of Nordenskiold Land (Spitsbergen Archipelago), Izv., Atmos. Ocean. Phys., 2020, vol. 56, no. 8, pp. 1376–1400.
De Wever, A., Muylaert, K., Van der Gucht, K., Pirlot, S., Cocquyt, C., Descy, J.P., Plisnier, P.-D., and Vyverman, W., Bacterial community composition in Lake Tanganyika: Vertical and horizontal heterogeneity, Appl. Environ. Microbiol., 2005, vol. 71, no. 9, pp. 5029–5037.
Dojka, M.A., Hugenholtz, P., Haack, S.K., and Pace, N.R., Microbial diversity in a hydrocarbon and chlorinated-solvent-contaminated aquifer undergoing intrinsic bioremediation, Appl. Environ. Microbiol., 1998, no. 10, pp. 3869–3877.
Donachie, S.P., Hou, S., Lee, K.S., Riley, C.W., Pikina, A., Belisle, C., Kempe, S., Gregory, T.S., Bossuyt, A., Boerema, J., Liu, J., Freitas, T.A., Malahoff, A., and Alam, M., The Hawaiian Archipelago: A microbial diversity hotspot, Microb. Ecol., 2014, vol. 48, no. 4, pp. 509–520.
Drahota, P., Mikutta, C., Falteisek, L., Duchoslav, V., and Klementova, M., Biologically induced formation of realgar deposits in soil, Geochim. Cosmochim. Acta, 2017, vol. 218, pp. 237–256.
Dziga, D., Kokocinski, M., Barylski, J., Nowicki, G., Maksylewicz, A., Antosiak, A., Banaś, A.K., and Strzałka, W., Correlation between specific groups of heterotrophic bacteria and microcystin biodegradation in freshwater bodies of central Europe, FEMS Microbiol. Ecol., 2019, vol. 95, no. 11, id 162.
Elshahed, M.S., Youssef, N.H., Spain, A.M., Sheik, C., Najar, F.Z., Sukharnikov, L.O., Roe, B.A., Davis, J.P., Schloss, P.D., Bailey, V.L., and Krumholz, L.R., Novelty and uniqueness patterns of rare members of the soil biosphere, Appl. Environ. Microbiol., 2008, vol. 74, no. 17, pp. 5422–5428.
Fabisch, M., Freyer, G., Johnson, C.A., Buchel, G., Akob, D.M., Neu, T.R., and Kusel, K., Dominance of ‘Gallionella capsiferriformans’ and heavy metal association with Gallionella-like stalks in metal-rich pH 6 mine water discharge, Geobiology, 2016, vol. 14, no. 1, pp. 68–90.
Fadrosh, D.W., Ma, B., Gajer, P., Sengamalay, N., Ott, S., Brotman, R.M., and Ravel, J., An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform, Microbiome, 2014, vol. 2, no. 1, id 6.
Farrow, J.A.E., Lawson, P.A., Hippe, H., Gauglitz, U., and Collins, M.D., Phylogenetic evidence that the Gram-negative nonsporulating bacterium Tissierella (Bacteroides) praeacuta is a member of the Clostridium subphylum of the Gram-positive bacteria and description of Tissierella creatinini sp. nov., Syst. Evol. Microbiol., 1995, vol. 45, pp. 436–440.
Fendrich, C., Halovibrio variabilis gen. nov. sp. nov., Pseudomonas halophila sp. nov. and a new halophilic aerobic coccoid Eubacterium from Great Salt Lake, Utah, USA, Syst. Appl. Microbiol., 1988, vol. 11, no. 1, pp. 36–43.
Flynn, T.M., Sanford, R.A., Ryu, H., Bethke, C.M., Levine, A.D., Ashbolt, N.J., and Santo Domingo, J.W., Functional microbial diversity explains groundwater chemistry in a pristine aquifer, BMC Microbiol., 2013, vol. 13, no. 1, pp. 1–15.
Forman, S.L., Lubinski, D.J., Ingolfsson, O., Zeeberg, J.J., Snyder, J.A., Siegert, M.J., and Matishov, G.G., A review of postglacial emergence on Svalbard, Franz Josef Land and Novaya Zemlya, Northern Eurasia, Quat. Sci. Rev., 2004, vol. 23, pp. 1391–1434.
Gallego, V., Sanchez-Porro, C., Garcia, M.T., and Ventosa, A., Massilia aurea sp. nov., isolated from drinking water, Int. J. Syst. Appl. Microbiol., 2006, vol. 56, no. 10, pp. 2449–2453.
Ganzert, L., Bajerski, F., and Wagner, D., Bacterial community composition and diversity of five different permafrost-affected soils of Northeast Greenland, FEMS Microbiol. Ecol., 2014, vol. 89, no. 2, pp. 426–441.
Gilichinsky, D.A., Wilson, G.S., Friedmann, E.I., McKay, C.P., Sletten, R.S., Rivkina, E.M., Vishnivetskaya, T.A., Erokhina, L.G., Ivanushkina, N.E., Kochkina, G.A., Shcherbakova, V.A., Soina, V.S., Spirina, E.V., Vorobyova, E.A., Fyodorov-Davydov, D.G., et al., Microbial populations in Antarctic permafrost: Biodiversity, state, age and implication for astrobiology, Astrobiology, 2007, vol. 7, no. 2, pp. 275–311.
Glass, J.B., Ranjan, P., Kretz, C.B., Nunn, B.L., Johnson, A.M., McManus, J., and Stewart, F.J., Adaptations of Atribacteria to life in methane hydrates: Hot traits for cold life, bioRxiv, 2019, 536078. https://www. biorxiv.org/content/10.1101/536078v1.
Gleeson, D.F., Williamson, C., Grasby, S.E., Pappalardo, R.T., Spear, J.R., and Templeton, A.S., Low temperature S0 biomineralization at a supraglacial spring system in the Canadian High Arctic, Geobiology, 2011, vol. 9, no. 4, pp. 360–375.
Gołębiewski, M., Deja-Sikora, E., Cichosz, M., Tretyn, A., and Wróbel, B., 16S rDNA pyrosequencing analysis of bacterial community in heavy metals polluted soils, Microb. Ecol., 2014, vol. 67, no. 3, pp. 635–647.
Good, I.J., The population frequencies of species and the estimation of population parameters, Biometrika, 1953, vol. 40, pp. 237–264.
Gupta, R.S., Chander, P., and George, S., Phylogenetic framework and molecular signatures for the class Chloroflexi and its different clades; proposal for division of the class Chloroflexi class. nov. into the suborder Chloroflexineae subord. nov., consisting of the emended family Oscillochloridaceae and the family Chloroflexaceae fam. nov., and the suborder Roseiflexineae subord. nov., containing the family Roseiflexaceae fam. Nov., Antonie van Leeuwenhoek, 2012, vol. 103, no. 1, pp. 99–119.
Gustave, W., Yuan, Z.F., Sekar, R., Ren, Y.X., Liu, J.Y., Zhang, J., and Chen, Z., Soil organic matter amount determines the behavior of iron and arsenic in paddy soil with microbial fuel cells, Chemosphere, 2019, vol. 237, id 124459.
Hammer, Ø., Harper, D.A.T., and Ryan, P.D., PAST: Paleontological statistics software package for education and data analysis, Palaeontol. Electron., 2001, vol. 4, no. 1.
Hanson, B.T., Molecular microbial ecology of sediments and subsurface groundwater at a coal tar-contaminated waste site, Ph.D. Dissertation, New York: Cornell Univ., 2013.
Harms, C., Ludwig, U., and Andreesen, J.R., Sarcosine reductase of Tissierella creatinophila: Purification and characterization of its components, Arch. Microbiol., 1998, vol. 170, no. 6, pp. 442–450.
Hitchman, S.P., Darling, W.G., and Williams, G.M., Stable isotope ratios in methane containing gases in the United Kingdom, Tech. Rep. WE/89/30, 1990. http:// nora.nerc.ac.uk/id/eprint/502528/1/WE89030.pdf.
Hugerth, L.W., Wefer, H.A., Lundin, S., Jakobsson, H.E., Lindberg, M., Rodin, S., Engstrand, L., and Andersson, A.F., DegePrime, a program for degenerate primer design for broad-taxonomic-range PCR in microbial ecology studies, Appl. Environ. Microbiol., 2014, vol. 80, no. 16, pp. 5116–5123.
Humlum, O., Instanes, A., and Sollid, J.L., Permafrost in Svalbard: A review of research history, climatic background and engineering challenges, Polar Res., 2003, vol. 22, pp. 191–215.
Inceoglu, B., Bettaieb, A., Da Silva, C.A.T., Lee, K.S.S., Haj, F.G., and Hammock, B.D., Endoplasmic reticulum stress in the peripheral nervous system is a significant driver of neuropathic pain, Proc. Natl. Acad. Sci. U. S. A., 2015, vol. 112, no. 29, pp. 9082–9087.
Jansson, J.K. and Taş, N., The microbial ecology of permafrost, Nat. Rev. Microbiol., 2014, vol. 12, no. 6, pp. 414–425.
Jeon, C.O., Park, M., Ro, H.S., Park, W., and Madsen, E.L., The naphthalene catabolic (nag) genes of Polaromonas naphthalenivorans CJ2: Evolutionary implications for two gene clusters and novel regulatory control, Appl. Environ. Microbiol., 2006, vol. 72, no. 2, pp. 1086–1095.
Johnson, S.S., Hebsgaard, M.B., Christensen, T.R., Mastepanov, M., Nielsen, R., Munch, K., Brand, T., Gilbert, M.T.P., Zuber, M.T., Bunce, M., Rønn, R., Gilichinsky, D., Froese, D., and Willerslev, E., Ancient bacteria show evidence of DNA repair, Proc. Natl. Acad. Sci. U. S. A., 2007, vol. 104, pp. 14401–14405.
Jørgensen, B.B., Beulig, F., Egger, M., Petro, C., Scholze, C., and Røy, H., Organoclastic sulfate reduction in the sulfate-methane transition of marine sediments, Geochim. Cosmochim. Acta, 2019, vol. 254, pp. 231–245.
Kaden, R., Spröer, C., Beyer, D., and Krolla-Sidenstein, P., Rhodoferax saidenbachensis sp. nov., a psychrotolerant, very slowly growing bacterium within the family Comamonadaceae, proposal of appropriate taxonomic position of Albidiferax ferrireducens strain T118T in the genus Rhodoferax and emended description of the genus Rhodoferax, Int. J. Syst. Evol. Microbiol., 2014, vol. 64, no. 4, pp. 1186–1193.
Kaminski, M.A., Furmanczyk, E.M., Sobczak, A., Dziembowski, A., and Lipinski, L., Pseudomonas silesiensis sp. nov. strain A3T isolated from a biological pesticide sewage treatment plant and analysis of the complete genome sequence, Syst. and Appl. Microbiol., 2018, vol. 41, no. 1, pp. 13–22.
Karaevskaya, E.S., Demchenko, L.S., Demidov, N.E., Rivkina, E.M., Bulat, S.A., and Gilichinsky, D.A., Archaeal diversity in permafrost sediments of Bunger Hills Oasis and King George Island (Antarctica) according to the 16S rRNA gene sequencing, Microbiology, 2014, vol. 83, no. 4, pp. 398–406.
Karaevskaya, E.S., Demidov, N.E., Shmelev, D.G., Rivkina, E.M., and Bulat, S.A., Study of the bacterial communities in the Antarctic oases’ permafrost by means of culturing, Probl. Arkt. Antarkt., 2017, vol. 1, no. 2, pp. 27–42.
Khlebnikova, G.M., Gilichinskii, D.A., Fedorov-Davydov, D.G., and Vorob’eva, E.A., Quantitative evaluation of microorganisms in permafrost deposits and buried soils, Microbiology, 1990, vol. 59, no. 1, pp. 106–112.
Kielak, A., Pijl, A.S., Van Veen, J.A., and Kowalchuk, G.A., Differences in vegetation composition and plant species identity lead to only minor changes in soil-borne microbial communities in a former arable field, FEMS Microbiol. Ecol., 2008, vol. 63, no. 3, pp. 372–382.
Kim, D.U., Kim, S.G., Lee, H., Park, A.Y., and Ka, J.O., Oryzihumus soli sp. nov., isolated from soil and emended description of the genus Oryzihumus, Int. J. Syst. Evol. Microbiol., 2017, vol. 67, no. 10, pp. 3960–3964.
Konieczna, I., Wojtasik, B., Kwinkowski, M., Burska, D., Nowiński, K., Żarnowiec, P., and Kaca, W., Analysis of cultivable aerobic bacteria isolated from bottom sediments in the Wijdefjorden region, Spitsbergen, Pol. Polar Res., 2011, vol. 32, no. 2, pp. 181–195.
Korehi, H., Blöthe, M., and Schippers, A., Microbial diversity at the moderate acidic stage in three different sulfidic mine tailings dumps generating acid mine drainage, Res. Microbiol., 2014, vol. 165, no. 9, pp. 713–718.
Kuever, J., Rainey, F.A., and Widdel, F., Desulfovibrio, in Bergey’s Manual of Systematics of Archaea and Bacteria, New York: Wiley, 2015, pp. 1–17.
Kumar, S., Stecher, G., and Tamura, K., MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets, Mol. Biol. Evol., 2016, vol. 33, no. is. 7, pp. 1870–1874.
Kumar, S., Stecher, G., Knyaz, C., and Tamura, K., MEGA X: Molecular evolutionary genetics analysis across computing platforms, Mol. Biol. Evol., 2018, vol. 35, pp. 1547–1549.
Kwon, M., Kim, M., Priscu, J.C., Hong, S.G., Kim, S.J., and Kim, O.S., Bacterial biodiversity in permanently ice-covered lakes of the McMurdo Dry Valleys, Antarctica, in 15th International Symposium on Microbial Ecology, Seoul, 2014.
Lane, D.J., 16S/23S rRNA sequencing, in Nucleic Acid Techniques in Bacterial Systematics, Stackebrandt, E. and Goodfellow, M., Eds., Chichester, U.K.: Wiley, 1991, pp. 115–175.
Lee, Y.J., Romanek, C.S., and Wiegel, J., Desulfosporosinus youngiae sp. nov., a spore-forming, sulfate-reducing bacterium isolated from a constructed wetland treating acid mine drainage, Int. J. Syst. Evol. Microbiol., 2009, vol. 59, no. 11, pp. 2743–2746.
Magurran, A.E., Ecological Diversity and Its Measurement, London: Croom Helm, 1983; Moscow: Mir, 1992.
Mangerud, J., Radiocarbon dating of marine shells, including a discussion of apparent age of recent shells from Norway, Boreas, 1972, vol. 1, no. 2, pp. 143–172.
Merkel, A.Yu., Tarnovetskii, I.Yu., Podosokorskaya, O.A., and Toshchakov, S.V., Analysis of 16S rRNA primer systems for profiling of thermophilic microbial communities, Microbiology, 2019, vol. 88, no. 6, pp. 671–680.
Miller, C.B. and Wheeler, P.A., Biological Oceanography, New York: Wiley, 2012.
Mitzscherling, J., Winkel, M., Winterfeld, M., Horn, F., Yang, S., Grigoriev, M.N., Wagner, D., Overduin, P.P., and Liebner, S., The development of permafrost bacterial communities under submarine conditions, J. Geophys. Res.: Biogeosci., 2017, vol. 122, no. 7, pp. 1689–1704.
Mitzscherling, J., Horn, F., Winterfeld, M., Mahler, L., Kallmeyer, J., Overduin, P.P., Schirrmeister, L., Winkel, M., Grigoriev, M.N., Wagner, D., and Liebner, S., Microbial community composition and abundance after millennia of submarine permafrost warming, Biogeosciences, 2019, vol. 16, pp. 3941–3958.
Mollenhauer, G., Grotheer, H., Gentz, T., Bonk, E., and Hefter, J., Standard operation procedures and performance of the MICADAS radiocarbon laboratory at Alfred Wegener Institute (AWI), Germany, Nucl. Instrum. Methods Phys. Res., Sect. B, 2021, vol. 496, pp. 45–51.
Müller, A.L., De Rezende, J.R., Hubert, C.R., Kjeldsen, K.U., Lagkouvardos, I., Berry, D., Jørgensen, B.B., and Loy, A., Endospores of thermophilic bacteria as tracers of microbial dispersal by ocean currents, ISME J., 2014, vol. 8, no. 6, pp. 1153–1165.
Müller, O., Bang-Andreasen, T., White, IIIR.A., Elberling, B., Taş, N., Kneafsey, T., Jansson, J.K., and Øvreås, L., Disentangling the complexity of permafrost soil by using high resolution profiling of microbial community composition, key functions and respiration rates, Environ. Microbiol., 2018, vol. 20, no. 12, pp. 4328–4342.
Niu, J., Deng, J., **ao, Y., He, Z., Zhang, X., Van Nostrand, J.D., Liang, Y., Deng, Y., Liu, X., and Yin, H., The shift of microbial communities and their roles in sulfur and iron cycling in a copper ore bioleaching system, Sci. Rep., 2016, vol. 6, id 34744.
Nunoura, T., Hirai, M., Miyazaki, M., Kazama, H., Makita, H., Hirayama, H., Furushima, Y., Yamamoto, H., Imachi, H., and Takai, K., Isolation and characterization of a thermophilic, obligately anaerobic and heterotrophic marine Chloroflexi bacterium from a Chloroflexi-dominated microbial community associated with a Japanese shallow hydrothermal system, and proposal for Thermomarinilinea lacunofontalis gen. nov., sp. nov, Microbes Environ., 2013, vol. 28, no. 2, pp. 228–235.
Opel, T., Murton, J.B., Wetterich, S., Meyer, H., Ashastina, K., Günther, F., Grotheer, H., Mollenhauer, G., Danilov, P.P., Boeskorov, V., Savvinov, G.N., and Schirrmeister, L., Past climate and continentality inferred from ice wedges at Batagay megaslump in the Northern Hemisphere’s most continental region, Yana Highlands, interior Yakutia, Clim. Past, 2019, vol. 15, pp. 1443–1461.
Orsi, W.D., Ecology and evolution of seafloor and subseafloor microbial communities, Nat. Rev. Microbiol., 2018, vol. 16, no. 11, pp. 671–683.
Pascual, J., Blanco, S., García-López, M., García-Salamanca, A., Bursakov, S.A., Genilloud, O., Bills, J.F., Ramos, J.L., and van Dillewijn, P., Assessing bacterial diversity in the rhizosphere of Thymus zygis growing in the Sierra Nevada National Park (Spain) through culture-dependent and independent approaches, PLoS One, 2016, vol. 11, no. 1, id e0146558.
Pavlov, M.S., Lira, F., Martinez, J.L., Olivares-Pacheco, J., and Marshall, S.H., Pseudomonas fildesensis sp. nov., a psychrotolerant bacterium isolated from Antarctic soil of King George Island, South Shetland Islands, Int. J. Syst. Evol. Microbiol., 2020, vol. 70, no. 5, pp. 3255–3263. https://doi.org/10.1099/ijsem.0.004165
Peipoch, M., Jones, R., and Valett, H.M., Spatial patterns in biofilm diversity across hierarchical levels of river-floodplain landscapes, PLoS One, 2015, vol. 10, no. 12, id e0144303.
Perini, L., Gostinčar, C., and Gunde-Cimerman, N., Fungal and bacterial diversity of Svalbard subglacial ice, Sci. Rep., 2019, vol. 9, no. 1, pp. 1–15.
Pozzato, L., Cathalot, C., Berrached, C., Toussaint, F., Stetten, E., Caprais, J.C., Pastor, L., Olu, K., and Rabouille, C., Early diagenesis in the Congo deep-sea fan sediments dominated by massive terrigenous deposits: Part I. Oxygen consumption and organic carbon mineralization using a micro-electrode approach, Deep Sea Res., Part II, 2017, vol. 142, pp. 125–138.
Quéméneur, M., Erauso, G., Frouin, E., Zeghal, E., Vandecasteele, C., Ollivier, B., Tamburini, Ch., Garel, M., Menez, B., and Postec, A., Hydrostatic pressure helps to cultivate an original anaerobic bacterium from the Atlantis Massif subseafloor (IODP Expedition 357): Petrocella atlantisensis gen. nov. sp. nov., Front. Microbiol., 2019, vol. 10, id 1497.
Ravenschlag, K., Sahm, K., and Amann, R., Quantitative molecular analysis of the microbial community in marine Arctic sediments (Svalbard), Appl. Environ. Microbiol., 2001, vol. 67, no. 1, pp. 387–395.
Reddy, P.V.V., Rao, S.S.S.N., Pratibha, M.S., Sailaja, B., Kavya, B., Manorama, R.R., and Shivaji, S., Bacterial diversity and bioprospecting for cold-active enzymes from culturable bacteria associated with sediment from a melt water stream of Midtre Lovénbreen glacier, an Arctic glacier, Res. Microbiol., 2009, vol. 160, no. 8, pp. 538–546.
Reimer, P.J., Bard, E., Bayliss, A., Beck, J.W., Blackwell, P.G., Bronk Ramsey, C., Buck, C.E., Cheng, H., Edwards, R.L., Friedrich, M., Grootes, P.M., Guilderson, T.P., Haflidason, H., Hajdas, I., Hatté, C., et al., IntCal13 and MARINE13 radiocarbon age calibration curves 0–50000 years cal BP, Radiocarbon, 2013, vol. 55, no. 4, pp. 1869–1887.
Romaniuk, K., Ciok, A., Decewicz, P., Uhrynowski, W., Budzik, K., Nieckarz, M., and Dziewit, L., Insight into heavy metal resistome of soil psychrotolerant bacteria originating from King George Island (Antarctica), Polar Biol., 2018, vol. 41, no. 7, pp. 1319–1333.
Sahm, K. and Berninger, U.G., Abundance, vertical distribution, and community structure of benthic prokaryotes from permanently cold marine sediments (Svalbard, Arctic Ocean), Mar. Ecol. Prog. Ser., 1998, vol. 165, pp. 71–80.
Salvigsen, O. and Høgvard, K., Glacial history, Holocene shoreline displacement and palaeoclimate based on radiocarbon ages in the area of Bockfjorden, north-western Spitsbergen, Svalbard, Polar Res., 2005, vol. 25, no. 1, pp. 15–24.
Sánchez-Andrea, I., Stams, A.J., Amils, R., and Sanz, J.L., Enrichment and isolation of acidophilic sulfate-reducing bacteria from Tinto River sediments, Environ. Microbiol. Rep., 2013, vol. 5, no. 5, pp. 672–678.
Schimel, J., Balser, T.C., and Wallenstein, M., Microbial stress-response physiology and its implications for ecosystem function, Ecology, 2007, vol. 88, no. 6, pp. 1386–1394.
Senger, K., Brugmans, P., Grundvåg, S.-A., Jochmann, M., Nøttvedt, A., Olaussen, S., Skotte, A., and Smyrak-Sikora, A., Petroleum, coal and research drilling onshore Svalbard: A historical perspective, Norw. J. Geol., 2019, vol. 99, no. 3.
Shakirov, R.B., Chemical and isotopic characteristics of hydrocarbon gases from Mendeleev and Golovnin volcanoes, Kunashir Island, Geochem. Int., 2014, vol. 52, no. 3, pp. 247–259.
Singh, P., Singh, S.M., Singh, R.N., Naik, S., Roy, U., Srivastava, A., and Bölter, M., Bacterial communities in ancient permafrost profiles of Svalbard, Arctic, J. Basic Microbiol., 2017, vol. 57, no. 12, pp. 1018–1036.
Singh, S.K., Verma, P., Ramaiah, N., Chandrashekar, A.A., and Shouche, Y.S., Phylogenetic diversity of archaeal 16s rRNA and ammonia monooxygenase genes from tropical estuarine sediments on the central west coast of India, Res. Microbiol., 2010, vol. 161, no. 3, pp. 177–186.
Solov’eva, D.A., Savel’eva, L.A., Verkulich, S.R., and Zazovskaya, E.P., Postglacial changes in the natural environment in the area of the Barentsburg mine (West Spitsbergen Island), in Theory and Methods of Polar Science: Proc. of Int. Youth Sci. Conf. on Polar Geodesy, Glaciology, Hydrology and Geophysics, St. Petersburg, 2018, pp. 213–222.
Srinivas, T.N.R., Rao, S.N., Reddy, P.V.V., Pratibha, M.S., Sailaja, B., Kavya, B., and Shivaji, S., Bacterial diversity and bioprospecting for cold-active lipases, amylases and proteases, from bacteria of Kongsfjorden and Ny-Ålesund, Svalbard, Arctic, Curr. Microbiol., 2009, vol. 59, no. 5, pp. 537–547.
Steven, B., Briggs, G., McKay, Ch.P., Pollard, W.H., Greer, C.W., and Whyte, L.G., Characterization of the microbial diversity in permafrost sample from the Canadian high Arctic using culture-dependent and culture-independent methods, FEMS Microbiol. Ecol., 2007, pp. 513–523.
Steven, B., Pollard, W.H., Greer, C.W., and Whyte, L.G., Microbial diversity and activity through a permafrost/ground ice core profile from the Canadian high Arctic, Environ. Microbiol., 2008, vol. 10, no. 12, pp. 3388–3403.
Stieglmeier, M., Klingl, A., Alves, R.J., Rittmann, S.K.M., Melcher, M., Leisch, N., and Schleper, C., Nitrososphaera viennensis gen. nov., sp. nov., an aerobic and mesophilic, ammonia-oxidizing archaeon from soil and a member of the archaeal phylum Thaumarchaeota, Int. J. Syst. Evol. Microbiol., 2014, vol. 64, no. 8, id 2738.
Svendsen, J.I. and Mangerud, J., Holocene glacial and climatic variations on Spitsbergen, Svalbard, Holocene, 1997, vol. 7, no. 1, pp. 45–57.
Szpak, M.T., Monteys, X., O’Reilly, S., Simpson, A.J., Garcia, X., Evans, R.L., Allen, C.C.R., McNally, D.J., Countier-Murias, D., and Kelleher, B.P., Geophysical and geochemical survey of a large marine pockmark on the Malin Shelf, Ireland, Geochem., Geophys., Geosyst., 2012, vol. 13, no. 1.
Tamura, K. and Nei, M., Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees, Mol. Biol. Evol., 1993, vol. 10, pp. 512–526.
Taranik, A.A., Geochemical indicators of long-term coalbed methane production areas as an independent source of energy on the example of Donbas deposits, Gazov. Prom-st., 2017, vol. 755, no. 7, pp. 24–27.
Taş, N., Prestat, E., Wang, S., Wu, Y., Ulrich, C., Kneafsey, T., Tringe, S.G., Torn, M.S., Hubbard, S.S., and Jansson, J.K., Landscape topography structures the soil microbiome in Arctic polygonal tundra, Nat. Commun., 2018, vol. 9, no. 1, pp. 1–13.
Teske, A., Durbin, A., Ziervogel, K., Cox, C., and Arnosti, C., Microbial community composition and function in permanently cold seawater and sediments from an Arctic fjord of Svalbard, Appl. Environ. Microbiol., 2011, vol. 77, no. 6, pp. 2008–2018.
Tischer, K., Kleinsteuber, S., Schleinitz, K.M., Fetzer, I., Spott, O., Stange, F., Lohse, U., Franz, J., Neumann, F., Gerling, S., Schmind, Ch., Hasselwander, E., Harms, H., and Wendeberg, A., Microbial communities along biogeochemical gradients in a hydrocarbon-contaminated aquifer, Environ. Microbiol., 2013, vol. 15, no. 9, pp. 2603–2615.
Tuorto, S.J., Darias, P., McGuinness, L.R., Panikov, N., Zhang, T., Häggblom, M.M., Kerkhof, L.J., Bacterial genome replication at subzero temperatures in permafrost, ISME J., 2014, vol. 8, no. 1, pp. 139–149.
Vázquez, S., Monien, P., Minetti, R.P., Jürgens, J., Curtosi, A., Primitz, J.V., Frikenhaus, S., Abele, D., Mac Cormack,W., and Helmke, E., Bacterial communities and chemical parameters in soils and coastal sediments in response to diesel spills at Carlini Station, Antarctica, Sci. Total Environ., 2017, vol. 605, pp. 26–37.
Vishnivetskaya, T., Kathariou, S., McGrath, J., Gilichinsky, D., and Tiedje, J.M., Low-temperature recovery strategies for the isolation of bacteria from ancient permafrost sediments, Extremophiles, 2000, vol. 4, no. 3, pp. 165–173.
Wang, F., Gai, Y., Chen, M., and **ao, X., Arthrobacter psychrochitiniphilus sp. nov., a psychrotrophic bacterium isolated from Antarctica, Int. J. Syst. Evol. Microbiol., 2009, vol. 59, pp. 2759–2762.
Wartiainen, I., Hestnes, A.G., McDonald, I.R., and Svenning, M.M., Methylobacter tundripaludum sp. nov., a methane-oxidizing bacterium from Arctic wetland soil on the Svalbard islands, Norway (78°N), Int. J. Syst. Evol. Microbiol., 2006, vol. 56, no. 1, pp. 109–113.
Watanabe, T., Kojima, H., and Fukui, M., Identity of major sulfur-cycle prokaryotes in freshwater lake ecosystems revealed by a comprehensive phylogenetic study of the dissimilatory adenylylsulfate reductase, Sci. Rep., 2016, vol. 6, id 36262.
Weber, Y., Damsté, J.S.S., Zopfi, J., De Jonge, C., Gilli, A., Schubert, C.J., Lepori, F., Lehmann, M.F., and Niemann, H., Redox-dependent niche differentiation provides evidence for multiple bacterial sources of glycerol tetraether lipids in lakes, Proc. Natl. Acad. Sci. U. S. A., 2018, vol. 115, no. 43, pp. 10926–10931.
Wegner, C.E. and Liesack, W., Unexpected dominance of elusive Acidobacteria in early industrial soft coal slags, Front. Microbiol., 2017, vol. 8, id 1023.
Wei, N. and Finneran, K.T., Microbial community analyses of three distinct, liquid cultures that degrade methyl tert-butyl ether using anaerobic metabolism, Biodegradation, 2009, vol. 20, no. 5, pp. 695–707.
Wilhelm, R.C., Hanson, B.T., Chandra, S., and Madsen, E., Community dynamics and functional characteristics of naphthalene-degrading populations in contaminated surface sediments and hypoxic/anoxic groundwater, Environ. Microbiol., 2018, vol. 20, no. 10, pp. 3543–3559.
Yamada, T., Imachi, H., Ohashi, A., Harada, H., Hanada, S., Kamagata, Y., and Sekiguchi, Y., Bellilinea caldifistulae gen. nov., sp. nov. and Longilinea arvoryzae gen. nov., sp. nov., strictly anaerobic, filamentous bacteria of the phylum Chloroflexi isolated from methanogenic propionate-degrading consortia, Int. J. Syst. Evol. Microbiol., 2007, vol. 57, no. 10, pp. 2299–2306.
Yudovich Yu.E. and Ketris, M.P., Carbon isotope ratios in the stratosphere and biosphere, Biosfera, 2010, vol. 2, no. 2, pp. 231–247.
Zeglin, L.H., Wang, B., Waythomas, C., Rainey, F., and Talbot, S.L., Organic matter quantity and source affects microbial community structure and function following volcanic eruption on Kasatochi Island, Alaska, Environ. Microbiol., 2016, vol. 18, no. 1, pp. 146–158.
Zeng, Y., Selyanin, V., Lukeš, M., Dean, J., Kaftan, D., Feng, F., and Koblížek, M., Characterization of the microaerophilic, bacteriochlorophyll a-containing bacterium Gemmatimonas phototrophica sp. nov., and emended descriptions of the genus Gemmatimonas and Gemmatimonas aurantiaca, Int. J. Syst. Evol. Microbiol., 2015, vol. 65, no. 8, pp. 2410–2419.
Zeng, Y.-X., Yu, Y., Liu, Y., and Li, H.-R., Psychrobacter glaciei sp. nov., isolated from the ice core of an Arctic glacier, Int. J. Syst. Evol. Microbiol., 2016, vol. 66, pp. 1792–1798.
Zeng, Y. and Koblížek, M., Phototrophic Gemmatimonadetes: A new “purple” branch on the bacterial tree of life, in Modern Topics in the Phototrophic Prokaryotes, Springer, 2017, pp. 163–192.
Zhang, D.C., Schumann, P., Liu, H.C., **n, Y.H., Zhou, Y.G., Schinner, F., and Margesin, R., Arthrobacter alpinus sp. nov., a psychrophilic bacterium isolated from Alpine soil, Int. J. Syst. Evol. Microbiol., 2010, vol. 60, no. 9, pp. 2149–2153.
ACKNOWLEDGMENTS
The authors thank E.A. Vorobyova (Lomonosov Moscow State University) for help in organizing experimental work on the cultivation of permafrost samples; A.Yu. Merkel (Vinogradsky Institute of Microbiology, RAS) for performing NGS analysis and consultation on the method; M.Y. Cherbunina and D.G. Shmelev for consultation on the properties of methane in permafrost.
Funding
The research has been supported by the grant from the Russian Science Foundation for the project no. 19-77-10066 (to Nikita Demidov) with respect to the next generation sequencing and by the Deutsche Forschungsgemeinschaft (DFG grant no. WE4390/7-1 to Sebastian Wetterich) with respect to the radiocarbon dating and TOC content. Field work at the cryosphere test polygon near Barentsburg was carried out as part of the Russian Arctic Expedition on Spitsbergen (RAES).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karaevskaya, E.S., Demidov, N.E., Kazantsev, V.S. et al. Bacterial Communities of Frozen Quaternary Sediments of Marine Origin on the Coast of Western Spitsbergen. Izv. Atmos. Ocean. Phys. 57, 895–917 (2021). https://doi.org/10.1134/S000143382108003X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S000143382108003X