Abstract
Type 2 diabetes mellitus (T2DM) can cause multidimensional cognitive deficits, among which working memory (WM) is usually involved at an early stage. However, the neural substrates underlying impaired WM in T2DM patients are still unclear. To clarify this issue, we utilized functional magnetic resonance imaging (fMRI) and independent component analysis to evaluate T2DM patients for alterations in brain activation and functional connectivity (FC) in WM networks and to determine their associations with cognitive and clinical variables. Twenty complication-free T2DM patients and 19 matched healthy controls (HCs) were enrolled and fMRI data were acquired during a block-designed 1-back WM task. The WM metrics of the T2DM patients showed no differences compared with those of the HCs, except for a slightly lower accuracy rate in the T2DM patients. Compared with the HCs, the T2DM patients demonstrated increased activation within their WM fronto-parietal networks and activation strength was significantly correlated with WM performance. The T2DM patients also showed decreased FC within and between their WM networks. Our results indicate that the functional integration of WM sub-networks was disrupted in the complication-free T2DM patients and that strengthened regional activity in fronto-parietal networks may compensate for the WM impairment caused by T2DM.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus (T2DM), a chronic metabolic disorder characterized by hyperglycemia due to insulin resistance, is growing in prevalence worldwide1. T2DM not only impairs metabolic-related bodily functions, but it is also associated with multidimensional cognitive deficits, such as deficits in execution, attention and memory2,3. Although cognitive decline may not be severe enough to affect the normal life quality of T2DM patients at an early stage2,4, compelling evidence suggests that T2DM can accelerate the aging process and increase the risk for dementia5,6. Recently, early detection and prevention have been considered the most promising strategies in co** with progressive dementia7; therefore, it is of great significance to identify the signs of early cognitive decline in T2DM patients before they develop dementia.
Neuroimaging methods can provide important insights into the neural mechanisms of cognitive impairment in patients with T2DM. For example, T2DM patients have been shown to exhibit distributed gray matter atrophy in the hippocampus, amygdala and prefrontal and parietal cortices and this atrophy might contribute to cognitive impairment8,9,10,11. T2DM is also frequently accompanied by brain vascular lesions, which have been associated with cognitive deficits4,12. Moreover, T2DM patients have been shown to exhibit alterations in regional spontaneous brain activity that are correlated with poor cognitive performance38,39. Furthermore, significant correlations were found between the activation amplitudes of fronto-parietal networks in T2DM patients and WM performance, suggesting that patients with stronger fronto-parietal activation possess better WM abilities. As a result, these correlation results further supported our hypothesis of the presence of compensatory mechanisms of brain activation during WM processing in T2DM patients.
Reduced functional connectivity in working memory networks
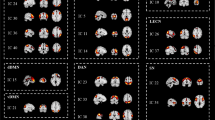
Our T2DM patients showed decreased intra-network FC in the bilateral lingual gyri of the visual network (IC 10), the vlPFC of the left fronto-parietal component (IC19) and the left IPL of the posterior parietal component (IC21). In addition, inter-network FC between the right fronto-parietal network (IC22) and visual network (IC10) were also decreased in T2DM patients.
Early studies reported that both the inferior frontal and the inferior parietal regions were involved in WM40. The vlPFC is closely related to both temporary information maintenance41,42,43 and comparison44 and is particularly critical for WM31,45. Recently, increased neural activity in the vlPFC was found in older adults compared with young adults during the performance of more complex tasks relative to a baseline; additionally, a higher BOLD signal in the vlPFC was shown to predict the efficiency of WM performance46. In diabetes, the vlPFC was considered to be vulnerably damaged19. IPL was frequently reported as playing a role in short-term memory storage which is an important content of 1-back working memory task47,48. In diabetes, decreased resting state FC in bilateral IPL was demonstratedhttp://www.fil.ion.ucl.ac.uk/spm/software/spm8). First, slice timing correction was performed to correct for the inter-slice time delay within each volume. Second, motion correction was estimated and corrected using rigid co-registration. Subjects who had head shift greater than 2.0 mm or rotation greater than 2.0° were excluded from the analyses. Third, images were spatially normalized into Montreal Neurological Institute (MNI) space using a standard EPI template provided by SPM8 and were resliced into a voxel-size of 3 mm × 3 mm × 3 mm. Finally, the data were spatially smoothed using a 6-mm full width at half-maximum Gaussian kernel.
Independent component analyses
Group spatial ICA was applied using GIFT software (http://icatb.sourceforge.net/, version 2.0d) to decompose the preprocessed fMRI images into spatial ICs as follows. First, 23 ICs were automatically estimated using the minimum description length criteria64. After that, a two-step principal component analysis was used to decompose the fMRI timecourses of the whole brain voxels into 23 principal components. This analysis was followed by group-level IC estimation using an Informax algorithm65. The most stable estimation of ICs was achieved by re-running the ICA analysis 100 times using the ICASSO method. Then, a spatial-temporal algorithm was used to back reconstruct the subject-level ICs from the group-level ICs. The spatial-temporal regression algorithm was introduced as following equations:


In which variable Y represents the group-level spatial components and variable X represents the preprocessed fMRI timecourses of a certain subject, TC represents the temporal components of this subjects and SC represents the spatial components of this subjects.
This step produced subject-level spatially IC maps (spatial components) as well as the featured time courses of these components (temporal components). Finally, the subject-specific spatial and temporal components were transformed into z-scores to create a normal distribution.
Component selection
We first used spatial correlation analysis to exclude ICs that were not contributed by gray matter. To accomplish this, we employed the following steps: first, maximum probability maps (MPMs) of gray matter, white matter and cerebral blood flow were generated using the tissue prior templates provided by SPM. A certain tissue class (gray matter, white matter, or cerebral blood flow) was defined for each voxel in the MPM based on the maximum probability among the three tissue prior templates. Then, the values for the spatial IC map and the MPM were rearranged to include one vector for each map and Pearson correlation analysis was performed to test the association between the vector of each spatial IC map and the vector of each MPM map. Finally, the highest spatial correlation coefficient of each IC was identified and the corresponding tissue class was retrieved. Following these steps, 15 ICs were identified as gray matter components. We further excluded 2 ICs that belong to the cerebellum. Then, the remaining 13 candidate components were further tested by a first-level canonical general linear model (GLM) to clarify whether the featured time course of a specific component was statistically related to the WM task. Specifically, the time course for each component of each subject was regressed against the design matrix of the WM task using GLM and canonical hemodynamic response function (HRF). The resulting β-estimate of each component represents the activation of this component in response to WM loading (1-back versus 0-back) in the subject. The β-estimates were further assessed using a second-level random effect one-sample t-test to clarify whether the mean β-estimate of each component was statistically significant (P < 0.05, Bonferroni correction). In total, eight of the 13 candidate components were found to be statistically associated with the WM task and were chosen for further analysis (Fig. 2).
WM-related intra-network and inter-network FC calculation
It should be noted that aforementioned subject-level spatial and temporal components were back-reconstructed using the full fMRI timecourses. Thus the components contained both 0-back and 1-back information. Because 0-back mainly reflects attention information but not the WM capacity, these subject-level components are not suitable for evaluate the WM-related FC changes in the T2DM. Thus, in order to obtain the spatial and temporal components that are specific for 1-back WM condition, we removed the timecourses that were abstained during the 0-back blocks from the raw fMRI data. We also corrected the HRF effect by delaying the task timing for 3 volumes (6 s). Then the remaining fMRI blocks were concatenate to form the timecourses that are specific for 1-back WM condition and they were considered as the variable X in the dual-regression function. Finally the spatial components and temporal components were scaled using Z-score.
The value of each voxel within a spatial component reflects the temporal coherence between the BOLD timecourses of each voxel and its temporal component. Thus, we termed the value of the spatial component as the intra-network functional connectivity. We also calculated the inter-network functional connectivity as the Pearson correlation between the temporal components of each IC pair, which were further Fisher r-to-z transformed to satisfy parametric statistics.
Statistical analyses
Activation analysis
Differences in global activation were tested by comparing the β-estimates of eight WM networks between the two groups using two-sample t-tests after controlling for the effects of age, gender and education (P < 0.05, Bonferroni correction). Finally, partial correlation coefficients were used to test the possible association between activation strength and clinical/cognitive variables after controlling for the effects of age, gender and education (P < 0.05).
Functional connectivity comparisons
A voxel-wise one-sample t-test was performed on the spatial components of the WM networks to recognize the spatial distribution pattern of the FC of each network. Multiple comparison corrections were made using family-wise error (FWE) correction (P < 0.05). The Cohen’s d effect size of each comparison was also calculated. Brain regions in each network showing statistically positive FC were binarized and used as mask for further intergroup comparisons. Intergroup differences in FC within eight networks were examined using two-sample t-tests after controlling for the effects of age, gender and education. Correction for multiple comparisons was performed using an AlphaSim algorithm, resulting in a corrected threshold of P < 0.05 at the cluster level (parameters: single voxel uncorrected P = 0.01, 1000 simulations, full width at half maximum = 6 mm, cluster connection radius r = 5 mm) and within the mask of the spatial distribution of each component. We further compared intergroup differences in FC between each pair of the eight WM networks using a two-sample t-test after controlling for the effects of age, gender and education (P < 0.05, uncorrected). The Cohen’s d effect size of each comparison was also calculated. Finally, partial correlation was used to assess possible associations between FC and clinical/cognitive variables after controlling for the effects of age, gender and education (P < 0.05).
Statistical analysis for demographic data
SPSS 21.0 (SPSS, Inc, Chiago.IL) was used to analyze demographic data. Shapiro-Wilk tests were performed to assess the distributions of demographic variables. Intergroup differences in demographic variables were tested either with Student’s t-test (normal distribution) or the Mann-Whitney U-test (non-normal distribution). Achi-squared (χ2) test was used to assess intergroup differences in gender. The significance level was set as P < 0.05.
Additional Information
How to cite this article: Zhang, Y. et al. Altered brain activation and functional connectivity in working memory related networks in patients with type 2 diabetes: An ICA-based analysis. Sci. Rep. 6, 23767; doi: 10.1038/srep23767 (2016).
References
Shaw, J. E., Sicree, R. A. & Zimmet, P. Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87, 4–14, doi: 10.1016/j.diabres.2009.10.007 (2010).
Reijmer, Y. D., van den Berg, E., Ruis, C., Kappelle, L. J. & Biessels, G. J. Cognitive dysfunction in patients with type 2 diabetes. Diabetes Metab Res Rev 26, 507–519, doi: 10.1002/dmrr.1112 (2010).
Toth, C. Diabetes and neurodegeneration in the brain. Handb Clin Neurol 126, 489–511, doi: 10.1016/b978-0-444-53480-4.00035-7 (2014).
Manschot, S. M. et al. Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes 55, 1106–1113 (2006).
Biessels, G. J., Staekenborg, S., Brunner, E., Brayne, C. & Scheltens, P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 5, 64–74, doi: 10.1016/S1474-4422(05)70284-2 (2006).
Mayeda, E. R., Whitmer, R. A. & Yaffe, K. Diabetes and Cognition. Clin Geriatr Med 31, 101–115, doi: 10.1016/j.cger.2014.08.021 (2015).
Borson, S. et al. Improving dementia care: the role of screening and detection of cognitive impairment. Alzheimers Dement 9, 151–159, doi: 10.1016/j.jalz.2012.08.008 (2013).
Moran, C. et al. Brain atrophy in type 2 diabetes: regional distribution and influence on cognition. Diabetes Care 36, 4036–4042, doi: 10.2337/dc13-0143 (2013).
Kumar, A. et al. Gray matter prefrontal changes in type 2 diabetes detected using MRI. J Magn Reson Imaging 27, 14–19, doi: 10.1002/jmri.21224 (2008).
Hayashi, K. et al. Association of cognitive dysfunction with hippocampal atrophy in elderly Japanese people with type 2 diabetes. Diabetes Res Clin Pract 94, 180–185, doi: 10.1016/j.diabres.2011.07.002 (2011).
Hempel, R., Onopa, R. & Convit, A. Type 2 diabetes affects hippocampus volume differentially in men and women. Diabetes Metab Res Rev 28, 76–83, doi: 10.1002/dmrr.1230 (2012).
McCrimmon, R. J., Ryan, C. M. & Frier, B. M. Diabetes and cognitive dysfunction. Lancet 379, 2291–2299, doi: 10.1016/S0140-6736(12)60360-2 (2012).
**a, W. et al. Altered baseline brain activity in type 2 diabetes: a resting-state fMRI study. Psychoneuroendocrinology 38, 2493–2501, doi: 10.1016/j.psyneuen.2013.05.012 (2013).
Musen, G. et al. Resting-state brain functional connectivity is altered in type 2 diabetes. Diabetes 61, 2375–2379, doi: 10.2337/db11-1669 (2012).
Baddeley, A. Working memory: looking back and looking forward. Nat Rev Neurosci 4, 829–839, doi: 10.1038/nrn1201 (2003).
Baddeley, A. The fractionation of working memory. Proc Natl Acad Sci USA 93, 13468–13472 (1996).
Takeuchi, A. et al. Characteristics of neuropsychological functions in inpatients with poorly-controlled type 2 diabetes mellitus. J Diabetes Investig 3, 325–330, doi: 10.1111/j.2040-1124.2011.00170.x (2012).
Cox, D. J. et al. Relationships between hyperglycemia and cognitive performance among adults with type 1 and type 2 diabetes. Diabetes Care 28, 71–77 (2005).
Chen, Y. et al. Altered brain activation patterns under different working memory loads in patients with type 2 diabetes. Diabetes Care 37, 3157–3163, doi: 10.2337/dc14-1683 (2014).
He, X. S. et al. Hyperactivation of working memory-related brain circuits in newly diagnosed middle-aged type 2 diabetics. Acta Diabetol, doi: 10.1007/s00592-014-0618-7 (2014).
He, X. S. et al. Hyperactivation of working memory-related brain circuits in newly diagnosed middle-aged type 2 diabetics. Acta Diabetol 52, 133–142, doi: 10.1007/s00592-014-0618-7 (2015).
Gazzaley, A., Rissman, J. & D’Esposito, M. Functional connectivity during working memory maintenance. Cogn Affect Behav Neurosci 4, 580–599 (2004).
Hampson, M., Driesen, N. R., Skudlarski, P., Gore, J. C. & Constable, R. T. Brain connectivity related to working memory performance. J Neurosci 26, 13338–13343, doi: 10.1523/JNEUROSCI.3408-06.2006 (2006).
Calhoun, V. D., Adali, T., Pearlson, G. D. & Pekar, J. J. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp 14, 140–151 (2001).
Calhoun, V. D., Adali, T., Pearlson, G. D. & Pekar, J. J. Spatial and temporal independent component analysis of functional MRI data containing a pair of task-related waveforms. Hum Brain Mapp 13, 43–53 (2001).
van den Bosch, G. E. et al. Brain connectivity during verbal working memory in children and adolescents. Hum Brain Mapp 35, 698–711, doi: 10.1002/hbm.22193 (2014).
Yakushev, I. et al. Metabolic and structural connectivity within the default mode network relates to working memory performance in young healthy adults. Neuroimage 79, 184–190, doi: 10.1016/j.neuroimage.2013.04.069 (2013).
Brandt, C. L. et al. Working memory networks and activation patterns in schizophrenia and bipolar disorder: comparison with healthy controls. Br J Psychiatry 204, 290–298, doi: 10.1192/bjp.bp.113.129254 (2014).
Wong, C. G. & Stevens, M. C. The effects of stimulant medication on working memory functional connectivity in attention-deficit/hyperactivity disorder. Biol Psychiatry 71, 458–466, doi: 10.1016/j.biopsych.2011.11.011 (2012).
Zhou, H. et al. Impairments in cognition and resting-state connectivity of the hippocampus in elderly subjects with type 2 diabetes. Neurosci Lett 473, 5–10, doi: 10.1016/j.neulet.2009.12.057 (2010).
Owen, A. M., McMillan, K. M., Laird, A. R. & Bullmore, E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp 25, 46–59, doi: 10.1002/hbm.20131 (2005).
Nee, D. E. et al. A meta-analysis of executive components of working memory. Cereb Cortex 23, 264–282, doi: 10.1093/cercor/bhs007 (2013).
Hsu, J. L. et al. Microstructural white matter abnormalities in type 2 diabetes mellitus: a diffusion tensor imaging study. Neuroimage 59, 1098–1105, doi: 10.1016/j.neuroimage.2011.09.041 (2012).
Wakisaka, M. et al. Reduced regional cerebral blood flow in aged noninsulin-dependent diabetic patients with no history of cerebrovascular disease: evaluation by N-isopropyl-123I-p-iodoamphetamine with single-photon emission computed tomography. J Diabet Complications 4, 170–174 (1990).
Reuter-Lorenz, P. A. & Cappell, K. A. Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Sci 17, 177–182 (2008).
Yetkin, F. Z., Rosenberg, R. N., Weiner, M. F., Purdy, P. D. & Cullum, C. M. FMRI of working memory in patients with mild cognitive impairment and probable Alzheimer’s disease. Eur Radiol 16, 193–206, doi: 10.1007/s00330-005-2794-x (2006).
Scheller, E., Minkova, L., Leitner, M. & Kloppel, S. Attempted and successful compensation in preclinical and early manifest neurodegeneration - a review of task FMRI studies. Front Psychiatry 5, 132, doi: 10.3389/fpsyt.2014.00132 (2014).
Qiu, W. Q. et al. Executive dysfunction in homebound older people with diabetes mellitus. J Am Geriatr Soc 54, 496–501, doi: 10.1111/j.1532-5415.2005.00632.x (2006).
Naorungroj, S. et al. Cross-sectional associations of oral health measures with cognitive function in late middle-aged adults: a community-based study. J Am Dent Assoc 144, 1362–1371 (2013).
Baldo, J. V. & Dronkers, N. F. The role of inferior parietal and inferior frontal cortex in working memory. Neuropsychology 20, 529 (2006).
D’Esposito, M., Postle, B. R. & Rypma, B. Prefrontal cortical contributions to working memory: evidence from event-related fMRI studies. Exp Brain Res 133, 3–11 (2000).
Rypma, B., Prabhakaran, V., Desmond, J. E., Glover, G. H. & Gabrieli, J. D. Load-dependent roles of frontal brain regions in the maintenance of working memory. Neuroimage 9, 216–226, doi: 10.1006/nimg.1998.0404 (1999).
Veltman, D. J., Rombouts, S. A. & Dolan, R. J. Maintenance versus manipulation in verbal working memory revisited: an fMRI study. Neuroimage 18, 247–256 (2003).
Wolf, R. C., Vasic, N. & Walter, H. Differential activation of ventrolateral prefrontal cortex during working memory retrieval. Neuropsychologia 44, 2558–2563, doi: 10.1016/j.neuropsychologia.2006.05.015 (2006).
Jonides, J., Smith, E. E., Marshuetz, C., Koeppe, R. A. & Reuter-Lorenz, P. A. Inhibition in verbal working memory revealed by brain activation. Proc Natl Acad Sci USA 95, 8410–8413 (1998).
Saliasi, E., Geerligs, L., Lorist, M. M. & Maurits, N. M. Neural correlates associated with successful working memory performance in older adults as revealed by spatial ICA. PLoS One 9, e99250, doi: 10.1371/journal.pone.0099250 (2014).
Buchsbaum, B. R., Ye, D. & D’Esposito, M. Recency Effects in the Inferior Parietal Lobe during Verbal Recognition Memory. Front Hum Neurosci 5, 59, doi: 10.3389/fnhum.2011.00059 (2011).
Greve, A., Doidge, A. N., Evans, C. J. & Wilding, E. L. Functional neuroanatomy supporting judgments of when events occurred. J Neurosci 30, 7099–7104 (2010).
**a, W. et al. Disrupted resting-state attentional networks in T2DM patients. Sci Rep 5, 11148, doi: 10.1038/srep11148 (2015).
Harrison, S. A. & Tong, F. Decoding reveals the contents of visual working memory in early visual areas. Nature 458, 632–635, doi: 10.1038/nature07832 (2009).
Pasternak, T. & Greenlee, M. W. Working memory in primate sensory systems. Nat Rev Neurosci 6, 97–107, doi: 10.1038/nrn1603 (2005).
Tong, F. & Pratte, M. S. Decoding patterns of human brain activity. Annu Rev Psychol 63, 483–509, doi: 10.1146/annurev-psych-120710-100412 (2012).
Coull, J., Frith, C., Frackowiak, R. S. J. & Grasby, P. A fronto-parietal network for rapid visual information processing: a PET study of sustained attention and working memory. Neuropsychologia 34, 1085–1095 (1996).
Bressler, S. L., Tang, W., Sylvester, C. M., Shulman, G. L. & Corbetta, M. Top-down control of human visual cortex by frontal and parietal cortex in anticipatory visual spatial attention. J Neurosci 28, 10056–10061, doi: 10.1523/JNEUROSCI.1776-08.2008 (2008).
Roelfsema, P. R. The role of the different layers of primary visual cortex in working memory. J Via 15, 1406–1406 (2015).
Association, A. D. Diagnosis and classification of diabetes mellitus. Diabetes care 33, S62–S69 (2010).
Folstein, M. F., Folstein, S. E. & McHugh, P. R. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12, 189–198 (1975).
Zung, W. W. A rating instrument for anxiety disorders. Psychosomatics 12, 371–379 (1971).
Zung, W. W. A self-rating depression scale. Arch Gen Psychiatry 12, 63–70 (1965).
Fecteau, S. et al. Diminishing risk-taking behavior by modulating activity in the prefrontal cortex: a direct current stimulation study. J Neurosci 27, 12500–12505, doi: 10.1523/JNEUROSCI.3283-07.2007 (2007).
Engelmann, J. B., Moore, S., Monica Capra, C. & Berns, G. S. Differential neurobiological effects of expert advice on risky choice in adolescents and adults. Soc Cogn Affect Neurosci 7, 557–567, doi: 10.1093/scan/nss050 (2012).
Roder, B., Kusmierek, A., Spence, C. & Schicke, T. Developmental vision determines the reference frame for the multisensory control of action. Proc Natl Acad Sci USA 104, 4753–4758, doi: 10.1073/pnas.0607158104 (2007).
Fiehler, K., Burke, M., Bien, S., Roder, B. & Rosler, F. The human dorsal action control system develops in the absence of vision. Cereb Cortex 19, 1–12, doi: 10.1093/cercor/bhn067 (2009).
Li, Y. O., Adalı, T. & Calhoun, V. D. Estimating the number of independent components for functional magnetic resonance imaging data. Hum Brain Mapp 28, 1251–1266 (2007).
Bell, A. J. & Sejnowski, T. J. An information-maximization approach to blind separation and blind deconvolution. Neural Comput 7, 1129–1159 (1995).
Author information
Authors and Affiliations
Contributions
Y.Z., W.Q. and Q.Z. designed the research. Y.Z., C.L., H.Z., X.Z., S.L. and Q.Z. performed the research. C.N. was involved in clinical assessment. Y.Z., S.L., W.Q. and Q.Z. analyzed the data. Y.Z., S.L., W.Q. and Q.Z. wrote the paper. Q.Z. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhang, Y., Lu, S., Liu, C. et al. Altered brain activation and functional connectivity in working memory related networks in patients with type 2 diabetes: An ICA-based analysis. Sci Rep 6, 23767 (2016). https://doi.org/10.1038/srep23767
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep23767
- Springer Nature Limited
This article is cited by
-
Brainstem network connectivity with mid-anterior insula predicts lower systolic blood pressure at rest in older adults with hypertension
Journal of Human Hypertension (2021)
-
Cerebral Biochemical Effect of Pregabalin in Patients with Painful Diabetic Neuropathy: A Randomized Controlled Trial
Diabetes Therapy (2018)
-
Piracetam Facilitates the Anti-Amnesic but not Anti-Diabetic Activity of Metformin in Experimentally Induced Type-2 Diabetic Encephalopathic Rats
Cellular and Molecular Neurobiology (2017)
-
Altered Intranetwork and Internetwork Functional Connectivity in Type 2 Diabetes Mellitus With and Without Cognitive Impairment
Scientific Reports (2016)