Abstract
The MoS2 and reduced graphite oxide (rGO) composite has attracted intensive attention due to its favorable performance as hydrogen evolution reaction (HER) catalyst, but still lacking is the theoretical understanding from a dynamic perspective regarding to the influence of electron transfer, as well as the connection between conductivity and the promoted HER performance. Based on the first-principles calculations, we here clearly reveal how an excess of negative charge density affects the variation of Gibbs free energy (ΔG) and the corresponding HER behavior. It is demonstrated that the electron plays a crucial role in the HER routine. To verify the theoretical analyses, the MoS2 and reduced graphite oxide (rGO) composite with well defined 3-dimensional configuration was synthesized via a facile one-step approach for the first time. The experimental data show that the HER performance have a direct link to the conductivity. These findings pave the way for a further develo** of 2-dimension based composites for HER applications.
Similar content being viewed by others
Introduction
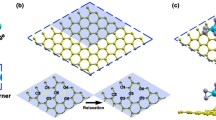
In recent years, the demands for the renewable and clean energy resources gradually become urgent for the growing problems of environmental pollution. Hydrogen, as a clean and efficient fuel source, has been vigorously pursued as a promising candidate for future energy carrier. The traditional way to produce hydrogen involving CO2 release and the high temperature reaction condition will be phased out gradually for the related disadvantages26. Figure 1f shows the average electrostatic potential of the pristine MoS2 and MoS2/graphene composite, in which two oscillation patterns of the average potential correspond to the periodicity of the supercell. The averaged potential for the pristine MoS2 and MoS2/graphene composite are −5.47 and −5.91 eV, respectively. It clearly shows that the averaged potential of composite structure is lower than that of pristine MoS2, implying an easier transfer of electrons form electrode to MoS2 in the direction perpendicular to the MoS2 basal plane for the MoS2/graphene composite and an increase of hop** efficiency for electrons in the vertical direction. Therefore, based on the above analyses, it is safe to conclude that an excess of negative charge density can form for the MoS2/graphene composite due to the achievement of an increased hop** efficiency for the composite structure when in the corresponding electrochemical processes.
The main feature of MoS2/graphene composite as analyzed above is that electrons can transfer from graphene to MoS2 directionally and the increased hop** efficiency of the system can form an excessive negative charge density. This motivates us to further study what role the excessive negative charge plays other than the enhanced conductivity. The considered atomic structures of basal plane, Mo and S edges are shown in the insert figures of (b–d). One thing to note is that the Mo edge is terminated with single S atom and that the Mo edge is not just a simple truncation of the bulk MoS2 structure27. The Mo-edge termination with a single S atom matches that of the 50% sulphur covered Mo edge previously observed in model catalysts and predicted by DFT28. It is commonly accepted that the criterion for a good HER catalyst is that the Gibbs free energy of adsorbed H is close to the thermo-neutral (i.e., ΔG ≈ 0). Thermodynamically, if the hydrogen adsorption is endothermic, the generation of surface H* would be hindered; while it is too exothermic, the removal of H* to form H2 would be difficultFull size image
Figure 3e,f show the SEM images of the synthesized MG3 nanoflowers composed by freely distributed petals. It presents a clear change in comparison with the pristine MoS2. The insert Fig. 3b,f of the two structures have the same scale, while the flower-like structures in Fig. 3f are significant more than that of pristine MoS2 and grown in a more free and loose way. As analyzed above, the ΔG of M-edge is more closer to zero than that of S-edge and basal site. The sharply increased petals can thus provide much more activity edges and enhance the HER performance. The MoS2 in these hybrid agglomerates are mainly consisted by limited-layer MoS2 structures and tightly coupled with underlying rGO sheets. In contrast, the TEM image of the MG3 nanostructure shown in Fig. 3g presents scattered and mild appearance of MoS2 grown on rGO sheets characterized by varied brightness. These results show that the incorporation of rGO can dramatically suppress the stacking of MoS2 to a great extent during its growth progress. Next, the crystallinities of the pristine MoS2 and MG3 composite are further characterized by FFT patterns. The FFT pattern of pristine MoS2 shown in the insert of Fig. 3d with bright spots denotes highly crystalline property and indexes definitely to MoS2. As for the MG3 composite shown in Fig. 3h, two sets of FFT patterns clearly demonstrate the coexistence of rGO and MoS2 along (002) direction. One thing to note here is that the FFT pattern of MoS2 in MG3 composite turns into arcs, which is clearly different from the well-defined crystal structure of pristine MoS2 and this indicates the formation of a quasi-crystalline structure of MG3 composite. We consider the nucleation mechanism on graphene was affected by oxygenated functional groups of GO. In the process of the GO reduced to rGO, MoS2 might grow on these sites. Graphene itself is 2D structured, while MoS2 has the nature of growing into flower. Both sides result in the confined growth of the corresponding MoS2 structure and form this special morphology and the petals are almost parallel to the graphene. Figure 3i further provides the direct evidence of the coexistence of hexagonal MoS2 and rGO. The upper left fringes of d = 0.62 nm is in agreement with the (002) plane of MoS2, indicating the synthesized petals grow in a crystallization way. The lower right exhibits lattice fringes of 0.34 nm which can be well indexed to (002) plane of 2H graphene (PDF#41-1487). It is apparent that the heterostructure formed between MoS2 and rGO in the MG3 composite. From the elemental map** of Fig. 3j for MG3, it can be seen that the extensive distribution of S, Mo and C. In brief, the modified MoS2 nanoflowers are clearly showed from Fig. 3e–i and the SEM/TEM analyses of the MG3 composite prove the formation of the corresponding 3D hierarchical architecture.
Figure 4a shows that three peaks of pristine MoS2 XRD spectrum are observed, indicating the crystallized MoS2 can be obtained through the hydrothermal method at 180 °C with the absence of GO. The three diffraction peaks around 2θ = 14°, 34° and 57° index to (002), (100) and (110) plane of MoS2 (JCPDS#37-1492), respectively. The four composites show a progressive crystallinity degree. For MG1-3, the broad peak 1 corresponds to the diffraction of MoS230, while the peak 2 around 24° attributes to rGO sheets31. Compared with MG3, the pristine MoS2 synthesized through the same conditions shows a better crystallinity and this is also accord with the above FFT observation. The significantly decreased diffraction peaks of MoS2 for MG3 imply that the incorporation of rGO can restrain the aggregation of natural stacked MoS2 during hydrothermal process to a certain degree, leading to a poor crystallinity with a few-layered MoS2 nanosheet. Previous reports have proven that the amorphous MoS2 presented higher HER activity than that of crystalline MoS2 within a given range since the amorphous structure can provide a mass of exposed edges32. It is speculated that the MoS2 with restrained crystallinity synthesized here can also provide ample edges as active sites due to their confined environment based on rGO network. A clear trend for MG composites is that the intensities of all diffraction peaks of MoS2 significantly increase with the increment of temperature and this also accompanies with the appearance of a new peak around 24° ascribed to rGO sheets. Based on the above analyses, it is credible to conclude that the synthesized MG composites at different temperatures have a progressive crystallinity.
Figure 4b displays two peaks at 382 and 407 cm−1 corresponding to the E12g and A1g vibrational modes for pristine MoS2, respectively. It has been reported that the frequency of A1g mode would present a red-shift as the layer number of MoS2 decreases33. As shown in Fig. 4b, a slightly red-shift of A1g mode is observed for MG3 composite in comparison with pristine MoS2, implying a decreased layer number after combination with rGO, which is also consistent with the above TEM and XRD analyses. The intensities of these two vibrational modes reflect the terminated structure of MoS2 and a lower intensity of E12g compared to A1g is associated with an edge-terminated characteristic34. At these sites, many unsaturated sulfur atoms serve as active sites and contribute to the improved HER activity. Besides, the Raman spectrum of MG3 shows two other prominent bands at 1351 and 1586 cm−1. The two bands correspond very well to the D and G-band of graphene. As previously reported, intensity ratio ID/IG is the quality indication of graphene35. The nearly 1 value of ID/IG agrees well with that of rGO, implying GO was indeed reduced to rGO with some defects or/and disordered structures36.
The chemical states of Mo and S in the pristine and MG3 are further investigated by XPS. The XPS spectrum of Mo 3d in pristine MoS2 is exhibited in Fig. 4c. Three peaks can be obtained after deconvolution. Among those 226.1 eV corresponds to S 2s of MoS2. The two main peaks of Mo 3d5/2 (229.3 eV) and Mo 3d3/2 (232.4 eV) are characteristics of MoS2. Sulfur species of pristine MoS2 are shown in Fig. 4d. The binding energies of 162.0 and 163.2 eV correspond to the S 2p3/2 and S 2p1/2 of MoS2, respectively37. As for MG3 composite structure, the above binding energies are all slightly larger than that of pristine MoS2. This result indicates that there exists a certain interaction between MoS2 and rGO sheets in the composited structure, driven by a probable electrons transfer between firmly contacted MoS2 and rGO sheets. In the composite, the co-electron cloud could form between S atom and its adjacent carbon layer. The interaction of electrons between MoS2 and rGO could dramatically enhance the conductivity of the composite and is conducive to the corresponding HER process. Generally, there exists the strong Coulomb interaction between the inner electrons and nucleus in the atom, while the outer electrons can also apply the shielding effect to the inner electrons. Therefore, the decrease of electron concentration of outer electrons will lead to the reduction of the corresponding shielding effect and thus increase the binding energy of the inner electrons for the existence of penetration effect, resulting in the corresponding XPS peaks shift to a higher energy direction. Obviously, the above XPS surveys reveal that electrons can transfer form MoS2 to rGO, which is also consistent with the calculated results that the directional transfer of electrons from MoS2 to graphene network and a built-in electric field establishment directed from MoS2 to graphene. A further quantitative elements analyses by XPS reveal that the oxygen percentages decreased from 5.31, 4.83, 4.11 to 3.27% and the atomic ratio of S:Mo also reduced from 2.27, 2.23, 2.12 to 2.07 as the synthesis temperatures increasing for MG1 to 4. These changes mean that the synthesis temperatures have a direct link to the incorporated oxygen and the unsaturated sulfur atoms, which will inevitably influence the electronic structure of MoS2 and exert a direct influence on the HER activity. This will be discussed in details in the following sections.
Figure 5a,b show polarization curves and the corresponding Tafel plots, respectively. It is shown that Pt exhibits a near zero overpotential and has the highest HER activity compared with the other catalysts owing to a high Hads coverage. The HER process on the Pt surface generally follows the Volmer-Tafel mechanism as described below. The rate-limiting step is corresponding to recombination step and it has the Tafel slope of 31 mV/decade. The polarization curve of GCE is also plotted, while the trivial current indicates that the GCE is catalytically inactive for the HER. It can be seen that much lower onset overpotentials (η) are obtained for all MG catalysts compared to pristine MoS2. Among composite structures, MG3 presents the lowest onset η of about 110 mV, implying a more superior HER activity and the cathodic current density rises rapidly under a more negative potential. The optimal catalytic performance of MG3 composite is attributed to the synergetic effect of the optimal intrinsic conductivity and the excellent conductive network provided by rGO network. The corresponding Tafel plot indicates that the suitably crystallized MG3 possesses the smallest Tafel slope than the poorly crystallized MG1 and MG2 or highly crystallized MG4. This proves that the crystallinity of MoS2 should be controlled in a reasonable level for a higher HER performance, since a poor crystallinity leads to the decreased conductivity for the suppressed electron transfer along the basal surfaces, which ends in a weakened HER activity. However, even if the improved crystallization can enhance the conductivity with increase of temperature, the inherent incorporated oxygen and sulphur atoms will gradually decrease as analyzed by XPS. In this case, the decrease of the two atoms that play promotion roles in the corresponding HER routine will lead to a subdued intrinsic conductivity in reverse which will be discussed later. Hence, a moderate crystallinity degree with quasi-crystallinity structure is more beneficial for HER.
(a) Polarization curves obtained with various catalysts. (b) The corresponding Tafel plots. The linear regions of the Tafel plots are fitted into the Tafel equation (η = blog(j) + a, where b is the Tafel slope). (c) Nyquist plots of EIS for various electrocatalysts at the modified GCEs. (d) Stability test of the MG3 electrocatalyst. Negligible HER current was lost after 2000 cycles of CV. (e) Current densities for MG3 composite with different loadings at overpotentials of 200 and 300 mV. (f) Potential values for 2000 CV sweeps for pristine MoS2 and MG composite structures.
The kinetic of the HER depends upon the electrochemical conditions and the corresponding Tafel slope is often utilized to elucidate the dominant mechanisms involved in the HER routine. There are two possible reaction steps in acidic aqueous. First, the discharge reaction38,

second, the combination reaction:

or the desorption reaction:

Generally, a fast discharge reaction (1) followed by a rate-limiting combination reaction (2) leads to a Tafel slope of ∼30 mV dec−1. When (1) is fast and followed by a slow electrochemical desorption reaction (3), a Tafel slope of ∼40 mV dec−1 is obtained. If (1) is rate-limiting or the surface coverage is close to one, the Tafel slope is ∼120 mV dec−1. The Tafel slope values obtained from Fig. 5b are ∼96, ∼55, ∼51, ∼41 and ∼43 mV dec−1 for pristine and MG1 to 4 composites, respectively. It is noteworthy that the Tafel slope of ∼41 mV dec−1 for MG3 composite suggests that desorption is the rate-limiting step.
EIS can be further utilized to study the interface reactions and electrode kinetics of pristine MoS2 and MG composites in HER processes. The Nyquist plots of different structures are given in Fig. 5c. The MG3 electrode shows the smallest radius of semi-circle in the Nyquist plots, implying the lowest contact and charge-transfer resistance (Rct) for the MG3 electrode. The obtained semicircles are also in accord with the HER activity shown in Fig. 5a. It is known that a lower Rct corresponds to a faster reaction rate. In this case, a faster charge transfer during HER reaction with a moderate crystallinity and rGO incorporation contribute to a superior HER activity of the MG3 composite for the achievement of both structural and electronic synergistic effect for the prominent HER performance. We also cycled the catalysts continually for 2000 cycles to probe HER stability. In this process, the catalyst poisoning or delaminating from the electrode may cause a certain activity loss of catalytic. As shown in Fig. 5d, at the end of cycling, the current density of MG3 composite catalyst decreases slightly in comparison with initial value after the continual cycling, implying that the synthesized MG3 composite exhibits good long term stability of HER activity. The dependence of different MG3 loadings on GCE and the HER activity is shown in Fig. 5e. It is shown that the optimal loading of MG3 is 15 mL with the current densities of 66 and 191 mA cm−2 at 200 and 300 mV, respectively. Besides, the potential values of various structures recorded at the same current density of 5 mA cm−2 for 2000 sweeps are displayed in Fig. 5f. It can be seen that all MG composites show good durability with negligible increases of overpotential in comparison with pristine MoS2. We consider that the hybrid structures of MG can enhance the durability, which can efficiently hinder the degradation of catalysts in HER routines.