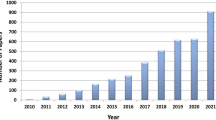
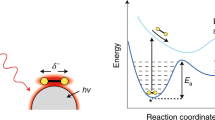
Abstract
Plasmon-mediated chemical reactions (PMCRs) are processes that make use of nanostructure-based surface plasmons as mediators to redistribute and convert photon energy in various time, space and energy scales, thereby driving chemical reactions by localizing photon, electronic and/or thermal energies. PMCRs can enhance the efficiency of an array of reactions, with potential advantages and unique features over thermochemistry, electrochemistry, photochemistry and photocatalysis. This Primer aims to give a general overview of the key points regarding PMCRs. Following the introduction of the main fundamental mechanism of plasmonic effects including enhanced electromagnetic near field, excited carriers and local heating on chemical reactions, the primary consideration and techniques for designing and constructing plasmonic catalysts are outlined. The typical methods used to characterize plasmonic catalysts and their properties are presented, as well as a discussion of PMCR mechanisms. Recent advances in PMCR application are also reviewed with some typical examples. Finally, the reproducibility and key optimization factors are emphasized, followed by a summary of future challenges and opportunities for the field.







Similar content being viewed by others
References
Zhan, C. et al. From plasmon-enhanced molecular spectroscopy to plasmon-mediated chemical reactions. Nat. Rev. Chem. 2, 216–230 (2018). This review summarizes the similarities and distinctive features of plasmon-enhanced molecular spectroscopy and PMCRs, and presents a comparison between PMCRs with traditional photochemical and thermochemical reactions.
Zhang, Y. et al. Surface-plasmon-driven hot electron photochemistry. Chem. Rev. 118, 2927–2954 (2018).
Baffou, G. & Quidant, R. Nanoplasmonics for chemistry. Chem. Soc. Rev. 43, 3898–3907 (2014).
Aslam, U., Rao, V. G., Chavez, S. & Linic, S. Catalytic conversion of solar to chemical energy on plasmonic metal nanostructures. Nat. Catal. 1, 656–665 (2018).
Christopher, P. & Moskovits, M. Hot charge carrier transmission from plasmonic nanostructures. Annu. Rev. Phys. Chem. 68, 379–398 (2017).
Hou, W. & Cronin, S. B. A review of surface plasmon resonance-enhanced photocatalysis. Adv. Funct. Mater. 23, 1612–1619 (2013).
Gargiulo, J., Berté, R., Li, Y., Maier, S. A. & Cortés, E. From optical to chemical hot spots in plasmonics. Acc. Chem. Res. 52, 2525–2535 (2019).
Maier, S. A. Plasmonics: Fundamentals and Applications (Springer, 2007). This work is an important reference book for fundamentals and applications of plasmonics.
Hartland, G. V. Optical studies of dynamics in noble metal nanostructures. Chem. Rev. 111, 3858–3887 (2011).
Schuller, J. A. et al. Plasmonics for extreme light concentration and manipulation. Nat. Mater. 9, 193–204 (2010).
Hutter, E. & Fendler, J. H. Exploitation of localized surface plasmon resonance. Adv. Mater. 16, 1685–1706 (2004).
Jain, P. K., Lee, K. S., El-Sayed, I. H. & El-Sayed, M. A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine. J. Phys. Chem. B 110, 7238–7248 (2006).
Amendola, V., Pilot, R., Frasconi, M., Maragò, O. M. & Iatì, M. A. Surface plasmon resonance in gold nanoparticles: a review. J. Phys. Condens. Matter 29, 203002 (2017).
Novotny, L. & van Hulst, N. Antennas for light. Nat. Photon. 5, 83–90 (2011).
Link, S. & El-Sayed, M. A. Spectral properties and relaxation dynamics of surface plasmon electronic oscillations in gold and silver nanodots and nanorods. J. Phys. Chem. B 103, 8410–8426 (1999).
Chen, H., Shao, L., Li, Q. & Wang, J. Gold nanorods and their plasmonic properties. Chem. Soc. Rev. 42, 2679–2724 (2013).
Noguez, C. Surface plasmons on metal nanoparticles: the influence of shape and physical environment. J. Phys. Chem. C 111, 3806–3819 (2007).
Brongersma, M. L., Halas, N. J. & Nordlander, P. Plasmon-induced hot carrier science and technology. Nat. Nanotech. 10, 25–34 (2015). This work is an excellent review of plasmon-induced hot carriers and their applications.
Khurgin, J. B. How to deal with the loss in plasmonics and metamaterials. Nat. Nanotech. 10, 2–6 (2015).
Huang, X., Jain, P. K., El-Sayed, I. H. & El-Sayed, M. A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 23, 217–228 (2008).
Baffou, G. & Quidant, R. Thermo‐plasmonics: using metallic nanostructures as nano‐sources of heat. Laser Photonics Rev. 7, 171–187 (2013).
Hao, F. et al. Symmetry breaking in plasmonic nanocavities: subradiant LSPR sensing and a tunable Fano resonance. Nano Lett. 8, 3983–3988 (2008).
Frischkorn, C. & Wolf, M. Femtochemistry at metal surfaces: nonadiabatic reaction dynamics. Chem. Rev. 106, 4207–4233 (2006).
Hartland, G. V., Besteiro, L. V., Johns, P. & Govorov, A. O. What’s so hot about electrons in metal nanoparticles? ACS Energy Lett. 2, 1641–1653 (2017).
Cho, G. C., Dekorsy, T., Bakker, H. J., Hövel, R. & Kurz, H. Generation and relaxation of coherent majority plasmons. Phys. Rev. Lett. 77, 4062–4065 (1996).
Inouye, H., Tanaka, K., Tanahashi, I. & Hirao, K. Ultrafast dynamics of nonequilibrium electrons in a gold nanoparticle system. Phys. Rev. B 57, 11334–11340 (1998).
Sundararaman, R., Narang, P., Jermyn, A. S., Goddard Iii, W. A. & Atwater, H. A. Theoretical predictions for hot-carrier generation from surface plasmon decay. Nat. Commun. 5, 5788 (2014). This work provides a theoretical description of plasmonic hot carrier generation in different plasmonic materials.
Govorov, A. O., Zhang, H., Demir, H. V. & Gun’ko, Y. K. Photogeneration of hot plasmonic electrons with metal nanocrystals: quantum description and potential applications. Nano Today 9, 85–101 (2014).
Manjavacas, A., Liu, J. G., Kulkarni, V. & Nordlander, P. Plasmon-induced hot carriers in metallic nanoparticles. ACS Nano 8, 7630–7638 (2014). This article presents a theoretical model to calculate the hot carrier production rate and energy distribution depending on the hot carrier lifetime and particle size.
Brown, A. M., Sundararaman, R., Narang, P., Goddard, W. A. & Atwater, H. A. Nonradiative plasmon decay and hot carrier dynamics: effects of phonons, surfaces, and geometry. ACS Nano 10, 957–966 (2016).
Baffou, G., Quidant, R. & Girard, C. Heat generation in plasmonic nanostructures: influence of morphology. Appl. Phys. Lett. 94, 153109 (2009).
Richardson, H. H., Carlson, M. T., Tandler, P. J., Hernandez, P. & Govorov, A. O. Experimental and theoretical studies of light-to-heat conversion and collective heating effects in metal nanoparticle solutions. Nano Lett. 9, 1139–1146 (2009).
Zhang, Z., Zhang, C., Zheng, H. & Xu, H. Plasmon-driven catalysis on molecules and nanomaterials. Acc. Chem. Res. 52, 2506–2515 (2019).
Nitzan, A. & Brus, L. E. Theoretical model for enhanced photochemistry on rough surfaces. J. Chem. Phys. 75, 2205–2214 (1981).
Chen, C. J. & Osgood, R. M. Direct observation of the local-field-enhanced surface photochemical reactions. Phys. Rev. Lett. 50, 1705–1708 (1983). Together with Nitzan and Brus (1981), this is the first paper predicting and demonstrating the enhanced photochemistry on rough surfaces.
Atwater, H. A. & Polman, A. Plasmonics for improved photovoltaic devices. Nat. Mater. 9, 205–213 (2010).
Liu, Z., Hou, W., Pavaskar, P., Aykol, M. & Cronin, S. B. Plasmon resonant enhancement of photocatalytic water splitting under visible illumination. Nano Lett. 11, 1111–1116 (2011).
Li, K. et al. Balancing near-field enhancement, absorption, and scattering for effective antenna–reactor plasmonic photocatalysis. Nano Lett. 17, 3710–3717 (2017).
Awazu, K. et al. A plasmonic photocatalyst consisting of silver nanoparticles embedded in titanium dioxide. J. Am. Chem. Soc. 130, 1676–1680 (2008).
Kazuma, E., Jung, J., Ueba, H., Trenary, M. & Kim, Y. Real-space and real-time observation of a plasmon-induced chemical reaction of a single molecule. Science 360, 521–526 (2018). This work discusses real-time and real-space map** of a PMCR.
Ingram, D. B. & Linic, S. Water splitting on composite plasmonic-metal/semiconductor photoelectrodes: evidence for selective plasmon-induced formation of charge carriers near the semiconductor surface. J. Am. Chem. Soc. 133, 5202–5205 (2011).
Yates, J. T. Photochemistry on TiO2: mechanisms behind the surface chemistry. Surf. Sci. 603, 1605–1612 (2009).
Ueno, K. et al. Nanoparticle plasmon-assisted two-photon polymerization induced by incoherent excitation source. J. Am. Chem. Soc. 130, 6928–6929 (2008).
Clavero, C. Plasmon-induced hot-electron generation at nanoparticle/metal-oxide interfaces for photovoltaic and photocatalytic devices. Nat. Photon. 8, 95–103 (2014).
Huang, Y.-F. et al. When the signal is not from the original molecule to be detected: chemical transformation of para-aminothiophenol on Ag during the SERS measurement. J. Am. Chem. Soc. 132, 9244–9246 (2010). This article presents the transformation of PATP on silver under excitation of surface plasmons.
Christopher, P., **n, H. & Linic, S. Visible-light-enhanced catalytic oxidation reactions on plasmonic silver nanostructures. Nat. Chem. 3, 467–472 (2011). This article demonstrates the plasmon-driven catalytic oxidation reactions on silver nanoparticles.
Mukherjee, S. et al. Hot electrons do the impossible: plasmon-induced dissociation of H2 on Au. Nano Lett. 13, 240–247 (2013). This article demonstrates the plasmon-mediated dissociation of H2 on gold nanoparticles.
Mubeen, S. et al. An autonomous photosynthetic device in which all charge carriers derive from surface plasmons. Nat. Nanotech. 8, 247–251 (2013). This article presents the whole water splitting mediated by plasmon-excited electrons and holes.
Ke, X. et al. Tuning the reduction power of supported gold nanoparticle photocatalysts for selective reductions by manipulating the wavelength of visible light irradiation. Chem. Commun. 48, 3509–3511 (2012).
Wu, K., Chen, J., McBride, J. R. & Lian, T. Efficient hot-electron transfer by a plasmon-induced interfacial charge-transfer transition. Science 349, 632–635 (2015). This work shows that plasmon-induced interfacial charge transfer transition is demonstrated by transient spectroscopy.
Boerigter, C., Aslam, U. & Linic, S. Mechanism of charge transfer from plasmonic nanostructures to chemically attached materials. ACS Nano 10, 6108–6115 (2016).
Kumar, P. V. et al. Plasmon-induced direct hot-carrier transfer at metal–acceptor interfaces. ACS Nano 13, 3188–3195 (2019).
Lee, S. A. & Link, S. Chemical interface dam** of surface plasmon resonances. Acc. Chem. Res. 54, 1950–1960 (2021).
Cortés, E. Activating plasmonic chemistry. Science 362, 28–29 (2018).
Baffou, G., Cichos, F. & Quidant, R. Applications and challenges of thermoplasmonics. Nat. Mater. 19, 946–958 (2020).
Mateo, D., Cerrillo, J. L., Durini, S. & Gascon, J. Fundamentals and applications of photo-thermal catalysis. Chem. Soc. Rev. 50, 2173–2210 (2021).
Fang, S. & Hu, Y. H. Thermo-photo catalysis: a whole greater than the sum of its parts. Chem. Soc. Rev. 51, 3609–3647 (2022).
Dubi, Y. & Sivan, Y. “Hot” electrons in metallic nanostructures — non-thermal carriers or heating? Light Sci. Appl. 8, 89 (2019).
Cao, L., Barsic, D. N., Guichard, A. R. & Brongersma, M. L. Plasmon-assisted local temperature control to pattern individual semiconductor nanowires and carbon nanotubes. Nano Lett. 7, 3523–3527 (2007).
Baffou, G. et al. Photoinduced heating of nanoparticle arrays. ACS Nano 7, 6478–6488 (2013).
Hogan, N. J. et al. Nanoparticles heat through light localization. Nano Lett. 14, 4640–4645 (2014).
Zhou, L. et al. 3D self-assembly of aluminium nanoparticles for plasmon-enhanced solar desalination. Nat. Photon. 10, 393–398 (2016).
Askes, S. H. C. & Garnett, E. C. Ultrafast thermal imprinting of plasmonic hotspots. Adv. Mater. 33, 2105192 (2021).
Zhan, C. et al. Plasmonic nanoreactors regulating selective oxidation by energetic electrons and nanoconfined thermal fields. Sci. Adv. 7, eabf0962 (2021).
Zhu, W. et al. Quantum mechanical effects in plasmonic structures with subnanometre gaps. Nat. Commun. 7, 11495 (2016).
Tame, M. S. et al. Quantum plasmonics. Nat. Phys. 9, 329–340 (2013).
**, J.-M. The Finite Element Method in Electromagnetics (Wiley, 2015).
Teixeira, F. L. FDTD/FETD methods: a review on some recent advances and selected applications. J. Microwaves Optoelectron. Electromagn. Appl. 6, 83–95 (2007).
Hohenester, U. & Trügler, A. MNPBEM — a MATLAB toolbox for the simulation of plasmonic nanoparticles. Comput. Phys. Commun. 183, 370–381 (2012).
Zhao, L., Zou, S., Hao, E. & Schatz, G.C. in Theory and Applications of Computational Chemistry Ch. 4 (eds Dykstra, C. E. et al.) 47–65 (Elsevier, 2005).
Narang, P., Sundararaman, R. & Atwater, H. A. Plasmonic hot carrier dynamics in solid-state and chemical systems for energy conversion. Nanophotonics 5, 96–111 (2016).
Rossi, T. P., Erhart, P. & Kuisma, M. Hot-carrier generation in plasmonic nanoparticles: the importance of atomic structure. ACS Nano 14, 9963–9971 (2020).
Baffou, G., Quidant, R. & Girard, C. Thermoplasmonics modeling: a Green’s function approach. Phys. Rev. B 82, 165424 (2010).
Linic, S., Chavez, S. & Elias, R. Flow and extraction of energy and charge carriers in hybrid plasmonic nanostructures. Nat. Mater. 20, 916–924 (2021). This work presents a comprehensive review of the flow and extraction of energy and charge carriers in hybrid plasmonic nanostructures.
Aslam, U., Chavez, S. & Linic, S. Controlling energy flow in multimetallic nanostructures for plasmonic catalysis. Nat. Nanotech. 12, 1000 (2017).
Yang, J., Wang, D., Han, H. & Li, C. Roles of cocatalysts in photocatalysis and photoelectrocatalysis. Acc. Chem. Res. 46, 1900–1909 (2013).
Zheng, Z., Tachikawa, T. & Majima, T. Plasmon-enhanced formic acid dehydrogenation using anisotropic Pd–Au nanorods studied at the single-particle level. J. Am. Chem. Soc. 137, 948–957 (2015).
Furube, A., Du, L., Hara, K., Katoh, R. & Tachiya, M. Ultrafast plasmon-induced electron transfer from gold nanodots into TiO2 nanoparticles. J. Am. Chem. Soc. 129, 14852–14853 (2007).
Bian, Z., Tachikawa, T., Zhang, P., Fujitsuka, M. & Majima, T. Au/TiO2 superstructure-based plasmonic photocatalysts exhibiting efficient charge separation and unprecedented activity. J. Am. Chem. Soc. 136, 458–465 (2013).
Zhang, Z. & Yates, J. T. Band bending in semiconductors: chemical and physical consequences at surfaces and interfaces. Chem. Rev. 112, 5520–5551 (2012).
Tagliabue, G. et al. Ultrafast hot-hole injection modifies hot-electron dynamics in Au/p-GaN heterostructures. Nat. Mater. 19, 1312–1318 (2020). This article demonstrates the ultrafast dynamics of hot holes in metal–semiconductor heterostructures.
Linsebigler, A. L., Lu, G. & Yates, J. T. Photocatalysis on TiO2 surfaces: principles, mechanisms, and selected results. Chem. Rev. 95, 735–758 (1995).
Jones, M. R., Osberg, K. D., Macfarlane, R. J., Langille, M. R. & Mirkin, C. A. Templated techniques for the synthesis and assembly of plasmonic nanostructures. Chem. Rev. 111, 3736–3827 (2011).
**a, Y., **ong, Y., Lim, B. & Skrabalak, S. E. Shape‐controlled synthesis of metal nanocrystals: simple chemistry meets complex physics? Angew. Chem. Int. Ed. 48, 60–103 (2009).
Tao, A. R., Habas, S. & Yang, P. Shape control of colloidal metal nanocrystals. Small 4, 310–325 (2008).
Lohse, S. E. & Murphy, C. J. The quest for shape control: a history of gold nanorod synthesis. Chem. Mater. 25, 1250–1261 (2013).
Yang, K., Yao, X., Liu, B. & Ren, B. Metallic plasmonic array structures: principles, fabrications, properties, and applications. Adv. Mater. 33, 2007988 (2021).
Grzelczak, M., Pérez-Juste, J., Mulvaney, P. & Liz-Marzán, L.M. in Colloidal Synthesis of Plasmonic Nanometals Ch. 6 (ed. Liz-Marzán, L.) 197–220 (Jenny Stanford, 2020).
Lin, H.-x et al. Supersaturation-dependent surface structure evolution: from ionic, molecular to metallic micro/nanocrystals. J. Am. Chem. Soc. 135, 9311–9314 (2013).
Wang, Z., Li, C. & Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 48, 2109–2125 (2019).
Zaera, F. Nanostructured materials for applications in heterogeneous catalysis. Chem. Soc. Rev. 42, 2746–2762 (2013).
Zhang, N. et al. Near-field dielectric scattering promotes optical absorption by platinum nanoparticles. Nat. Photon. 10, 473–482 (2016).
Zhang, W. et al. Large scale synthesis of pinhole-free shell-isolated nanoparticles (SHINs) using improved atomic layer deposition (ALD) method for practical applications. J. Raman Spectrosc. 46, 1200–1204 (2015).
Dutta, S. K., Mehetor, S. K. & Pradhan, N. Metal semiconductor heterostructures for photocatalytic conversion of light energy. J. Phys. Chem. Lett. 6, 936–944 (2015).
Sun, Z. et al. A general approach to the synthesis of gold–metal sulfide core–shell and heterostructures. Angew. Chem. Int. Ed. 48, 2881–2885 (2009).
Fan, F.-R. et al. Epitaxial growth of heterogeneous metal nanocrystals: from gold nano-octahedra to palladium and silver nanocubes. J. Am. Chem. Soc. 130, 6949–6951 (2008).
Seh, Z. W. et al. Janus Au–TiO2 photocatalysts with strong localization of plasmonic near-fields for efficient visible-light hydrogen generation. Adv. Mater. 24, 2310–2314 (2012).
Mokari, T., Sztrum, C. G., Salant, A., Rabani, E. & Banin, U. Formation of asymmetric one-sided metal-tipped semiconductor nanocrystal dots and rods. Nat. Mater. 4, 855–863 (2005).
Nørskov, J. K. et al. The nature of the active site in heterogeneous metal catalysis. Chem. Soc. Rev. 37, 2163–2171 (2008).
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Lin, Q. et al. Optical suppression of energy barriers in single molecule-metal binding. Sci. Adv. 8, eabp9285 (2022).
Modena, M. M., Rühle, B., Burg, T. P. & Wuttke, S. Nanoparticle characterization: what to measure? Adv. Mater. 31, 1901556 (2019).
Chee, S. W., Lunkenbein, T., Schlögl, R. & Cuenya, B. R. In situ and operando electron microscopy in heterogeneous catalysis — insights into multi-scale chemical dynamics. J. Phys. Condens. Matter 33, 153001 (2021).
Jans, H., Liu, X., Austin, L., Maes, G. & Huo, Q. Dynamic light scattering as a powerful tool for gold nanoparticle bioconjugation and biomolecular binding studies. Anal. Chem. 81, 9425–9432 (2009).
Liu, B.-J. et al. Extraction of absorption and scattering contribution of metallic nanoparticles toward rational synthesis and application. Anal. Chem. 87, 1058–1065 (2015).
Pestryakov, A. N. et al. Study of copper nanoparticles formation on supports of different nature by UV–Vis diffuse reflectance spectroscopy. Chem. Phys. Lett. 385, 173–176 (2004).
Li, Y., **g, C., Zhang, L. & Long, Y.-T. Resonance scattering particles as biological nanosensors in vitro and in vivo. Chem. Soc. Rev. 41, 632–642 (2012).
Hu, S. et al. Observing atomic layer electrodeposition on single nanocrystals surface by dark field spectroscopy. Nat. Commun. 11, 2518 (2020).
Novo, C., Funston, A. M. & Mulvaney, P. Direct observation of chemical reactions on single gold nanocrystals using surface plasmon spectroscopy. Nat. Nanotech. 3, 598–602 (2008). This article establishes a quantitative method of using scattering spectroscopy to probe the catalytic process at the single nanoparticle level.
Dombi, P. et al. Strong-field nano-optics. Rev. Mod. Phys. 92, 025003 (2020).
Esmann, M. et al. Vectorial near-field coupling. Nat. Nanotech. 14, 698–704 (2019).
Polman, A., Kociak, M. & García de Abajo, F. J. Electron-beam spectroscopy for nanophotonics. Nat. Mater. 18, 1158–1171 (2019).
Langer, J. et al. Present and future of surface-enhanced Raman scattering. ACS Nano 14, 28–117 (2020).
Schnell, M. et al. Controlling the near-field oscillations of loaded plasmonic nanoantennas. Nat. Photon. 3, 287–291 (2009).
Sheldon, M. T., van de Groep, J., Brown, A. M., Polman, A. & Atwater, H. A. Plasmoelectric potentials in metal nanostructures. Science 346, 828–831 (2014).
Chen, R., Fan, F., Dittrich, T. & Li, C. Imaging photogenerated charge carriers on surfaces and interfaces of photocatalysts with surface photovoltage microscopy. Chem. Soc. Rev. 47, 8238–8262 (2018).
Zhang, H. et al. Plasmon-induced interfacial hot-electron transfer directly probed by Raman spectroscopy. Chem 6, 689–702 (2020).
Zhan, C. et al. Interfacial construction of plasmonic nanostructures for the utilization of the plasmon-excited electrons and holes. J. Am. Chem. Soc. 141, 8053–8057 (2019).
Sambur, J. B. et al. Sub-particle reaction and photocurrent map** to optimize catalyst-modified photoanodes. Nature 530, 77–80 (2016).
Huang, Y.-F. et al. Microphotoelectrochemical surface-enhanced Raman spectroscopy: toward bridging hot-electron transfer with a molecular reaction. J. Am. Chem. Soc. 142, 8483–8489 (2020).
Schlather, A. E. et al. Hot hole photoelectrochemistry on Au@SiO2@Au nanoparticles. J. Phys. Chem. Lett. 8, 2060–2067 (2017).
Zhan, C. et al. Disentangling charge carrier from photothermal effects in plasmonic metal nanostructures. Nat. Commun. 10, 2671 (2019). This work presents the unique photoelectrochemical feature of plasmonic electrodes.
Reddy, H. et al. Determining plasmonic hot-carrier energy distributions via single-molecule transport measurements. Science 369, 423–426 (2020).
Zhang, X. et al. Plasmon-enhanced catalysis: distinguishing thermal and nonthermal effects. Nano Lett. 18, 1714–1723 (2018).
Zhang, Z. et al. Insights into the nature of plasmon-driven catalytic reactions revealed by HV-TERS. Nanoscale 5, 3249–3252 (2013).
Hu, S. et al. Quantifying surface temperature of thermoplasmonic nanostructures. J. Am. Chem. Soc. 140, 13680–13686 (2018).
Baffou, G., Kreuzer, M. P., Kulzer, F. & Quidant, R. Temperature map** near plasmonic nanostructures using fluorescence polarization anisotropy. Opt. Express 17, 3291–3298 (2009).
Wang, D. et al. Spatial and temporal nanoscale plasmonic heating quantified by thermoreflectance. Nano Lett. 19, 3796–3803 (2019).
Zhou, L. et al. Quantifying hot carrier and thermal contributions in plasmonic photocatalysis. Science 362, 69–72 (2018). This article uses light-dependent activation barriers to distinguish the hot carrier and thermal contributions in PMCRs, and has sparked a lot of discussion about temperature measurement in PMCR systems.
Brolo, A. G., Sanderson, A. C. & Smith, A. P. Ratio of the surface-enhanced anti-Stokes scattering to the surface-enhanced Stokes–Raman scattering for molecules adsorbed on a silver electrode. Phys. Rev. B 69, 045424 (2004).
Itoh, T. et al. Second enhancement in surface-enhanced resonance Raman scattering revealed by an analysis of anti-Stokes and Stokes Raman spectra. Phys. Rev. B 76, 085405 (2007).
Lin, K.-Q. et al. Plasmonic photoluminescence for recovering native chemical information from surface-enhanced Raman scattering. Nat. Commun. 8, 14891 (2017).
Desiatov, B., Goykhman, I. & Levy, U. Direct temperature map** of nanoscale plasmonic devices. Nano Lett. 14, 648–652 (2014).
Majumdar, A. Scanning thermal microscopy. Annu. Rev. Mater. Sci. 29, 505–585 (1999).
Suh, J. S., Jang, N. H., Jeong, D. H. & Moskovits, M. Adsorbate photochemistry on a colloid surface: phthalazine on silver. J. Phys. Chem. 100, 805–813 (1996).
Jeong, D. H., Jang, N. H., Suh, J. S. & Moskovits, M. Photodecomposition of diazanaphthalenes adsorbed on silver colloid surfaces. J. Phys. Chem. B 104, 3594–3600 (2000).
Zhan, C., Chen, X.-J., Huang, Y.-F., Wu, D.-Y. & Tian, Z.-Q. Plasmon-mediated chemical reactions on nanostructures unveiled by surface-enhanced Raman spectroscopy. Acc. Chem. Res. 52, 2784–2792 (2019).
van Schrojenstein Lantman, E. M., Deckert-Gaudig, T., Mank, A. J., Deckert, V. & Weckhuysen, B. M. Catalytic processes monitored at the nanoscale with tip-enhanced Raman spectroscopy. Nat. Nanotech. 7, 583–586 (2012).
Wang, Q. et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%. Nat. Mater. 15, 611–615 (2016).
Wang, Z. et al. Efficiency accreditation and testing protocols for particulate photocatalysts toward solar fuel production. Joule 5, 344–359 (2021).
Zhong, Y. et al. Plasmon-assisted water splitting using two sides of the same SrTiO3 single-crystal substrate: conversion of visible light to chemical energy. Angew. Chem. Int. Ed. 53, 10350–10354 (2014).
Shi, X. et al. Enhanced water splitting under modal strong coupling conditions. Nat. Nanotech. 13, 953–958 (2018).
Kale, M. J., Avanesian, T. & Christopher, P. Direct photocatalysis by plasmonic nanostructures. ACS Catal. 4, 116–128 (2014).
Christopher, P., **n, H., Marimuthu, A. & Linic, S. Singular characteristics and unique chemical bond activation mechanisms of photocatalytic reactions on plasmonic nanostructures. Nat. Mater. 11, 1044–1050 (2012).
Swearer, D. F. et al. Heterometallic antenna–reactor complexes for photocatalysis. Proc. Natl Acad. Sci. USA 113, 8916–8920 (2016). This work presents heterometallic antenna−reactor complexes with a plasmonic nanostructure and catalytic mediator for PMCRs.
Wang, C. et al. Endothermic reaction at room temperature enabled by deep-ultraviolet plasmons. Nat. Mater. 20, 346–352 (2021).
Kim, Y., Smith, J. G. & Jain, P. K. Harvesting multiple electron–hole pairs generated through plasmonic excitation of Au nanoparticles. Nat. Chem. 10, 763–769 (2018).
Govorov, A. O. & Richardson, H. H. Generating heat with metal nanoparticles. Nano Today 2, 30–38 (2007).
Han, X. X., Rodriguez, R. S., Haynes, C. L., Ozaki, Y. & Zhao, B. Surface-enhanced Raman spectroscopy. Nat. Rev. Methods Primers 1, 87 (2022).
Ding, S.-Y. et al. Nanostructure-based plasmon-enhanced Raman spectroscopy for surface analysis of materials. Nat. Rev. Mater. 1, 16021 (2016).
Wang, X., Huang, S.-C., Hu, S., Yan, S. & Ren, B. Fundamental understanding and applications of plasmon-enhanced Raman spectroscopy. Nat. Rev. Phys. 2, 253–271 (2020).
Boerigter, C., Campana, R., Morabito, M. & Linic, S. Evidence and implications of direct charge excitation as the dominant mechanism in plasmon-mediated photocatalysis. Nat. Commun. 7, 10545 (2016).
Liu, J. et al. Plasmonic hot electron-mediated hydrodehalogenation kinetics on nanostructured Ag electrodes. J. Am. Chem. Soc. 142, 17489–17498 (2020).
Bonn, M. et al. Phonon- versus electron-mediated desorption and oxidation of CO on Ru(0001). Science 285, 1042–1045 (1999). This article presents a comparison of phonon-mediated and electron-mediated chemical reaction.
Huang, S.-C. et al. Probing nanoscale spatial distribution of plasmonically excited hot carriers. Nat. Commun. 11, 4211 (2020). This article uses TERS to probe the nanoscale spatial distribution of plasmonically excited hot carriers.
Morton, S. M., Silverstein, D. W. & Jensen, L. Theoretical studies of plasmonics using electronic structure methods. Chem. Rev. 111, 3962–3994 (2011).
Wu, Q., Zhou, L., Schatz, G. C., Zhang, Y. & Guo, H. Mechanistic insights into photocatalyzed H2 dissociation on Au clusters. J. Am. Chem. Soc. 142, 13090–13101 (2020). This article uses DFT and TDDFT calculation to describe the physical picture of the mechanism of localized SPR-induced H2 dissociation on gold clusters.
Ma, H., Gao, F. & Liang, W. Plasmon resonance of isolated gold hollow nanoparticles and nanoparticle pairs: insights from electronic structure calculations. J. Phys. Chem. C 116, 1755–1763 (2012).
Long, R. & Prezhdo, O. V. Instantaneous generation of charge-separated state on TiO2 surface sensitized with plasmonic nanoparticles. J. Am. Chem. Soc. 136, 4343–4354 (2014).
Jensen, L., Aikens, C. M. & Schatz, G. C. Electronic structure methods for studying surface-enhanced Raman scattering. Chem. Soc. Rev. 37, 1061–1073 (2008).
Zhang, R. et al. How to identify plasmons from the optical response of nanostructures. ACS Nano 11, 7321–7335 (2017).
Bursi, L., Calzolari, A., Corni, S. & Molinari, E. Light-induced field enhancement in nanoscale systems from first-principles: the case of polyacenes. ACS Photonics 1, 1049–1058 (2014).
Zhang, P., **, W. & Liang, W. Size-dependent optical properties of aluminum nanoparticles: from classical to quantum description. J. Phys. Chem. C 122, 10545–10551 (2018).
Hammer, B., Morikawa, Y. & Nørskov, J. K. CO chemisorption at metal surfaces and overlayers. Phys. Rev. Lett. 76, 2141 (1996).
Calle-Vallejo, F., Loffreda, D., Koper, M. & Sautet, P. Introducing structural sensitivity into adsorption–energy scaling relations by means of coordination numbers. Nat. Chem. 7, 403–410 (2015).
Kresse, G., Gil, A. & Sautet, P. Significance of single-electron energies for the description of CO on Pt(111). Phys. Rev. B 68, 073401 (2003).
Ji, Y. & Luo, Y. Theoretical study on the mechanism of photoreduction of CO2 to CH4 on the anatase TiO2 (101) surface. ACS Catal. 6, 2018–2025 (2016).
Chu, W., Zheng, Q., Prezhdo, O. V. & Zhao, J. CO2 photoreduction on metal oxide surface is driven by transient capture of hot electrons: ab initio quantum dynamics simulation. J. Am. Chem. Soc. 142, 3214–3221 (2020).
Zhang, L. et al. Dynamics of photoexcited small polarons in transition-metal oxides. J. Phys. Chem. Lett. 12, 2191–2198 (2021).
Martirez, J. M. P. & Carter, E. A. Excited-state N2 dissociation pathway on Fe-functionalized Au. J. Am. Chem. Soc. 139, 4390–4398 (2017).
Yin, R. et al. Dissociative chemisorption of O2 on Al(111): dynamics on a correlated wave-function-based potential energy surface. J. Phys. Chem. Lett. 9, 3271–3277 (2018).
Zhang, X.-G. et al. Light driven mechanism of carbon dioxide reduction reaction to carbon monoxide on gold nanoparticles: a theoretical prediction. J. Phys. Chem. Lett. 12, 1125–1130 (2021).
Zhang, Y., Nelson, T., Tretiak, S., Guo, H. & Schatz, G. C. Plasmonic hot-carrier-mediated tunable photochemical reactions. ACS Nano 12, 8415–8422 (2018).
Gavnholt, J., Olsen, T., Engelund, M. & Schiøtz, J. Δ self-consistent field method to obtain potential energy surfaces of excited molecules on surfaces. Phys. Rev. B 78, 075441 (2008).
Olsen, T., Gavnholt, J. & Schiøtz, J. Hot-electron-mediated desorption rates calculated from excited-state potential energy surfaces. Phys. Rev. B 79, 035403 (2009).
Behler, J., Delley, B., Reuter, K. & Scheffler, M. Nonadiabatic potential-energy surfaces by constrained density-functional theory. Phys. Rev. B 75, 115409 (2007).
Wu, D.-Y. et al. Surface catalytic coupling reaction of p-mercaptoaniline linking to silver nanostructures responsible for abnormal SERS enhancement: a DFT study. J. Phys. Chem. C 113, 18212–18222 (2009).
Barone, V. et al. Computational molecular spectroscopy. Nat. Rev. Methods Primers 1, 38 (2021).
Neugebauer, J., Reiher, M., Kind, C. & Hess, B. A. Quantum chemical calculation of vibrational spectra of large molecules — Raman and IR spectra for buckminsterfullerene. J. Comput. Chem. 23, 895–910 (2002).
Wu, D.-Y. et al. Chemical enhancement effects in SERS spectra: a quantum chemical study of pyridine interacting with copper, silver, gold and platinum metals. J. Phys. Chem. C 112, 4195–4204 (2008).
Duan, S., Ai, Y.-J., Hu, W. & Luo, Y. Roles of plasmonic excitation and protonation on photoreactions of p-aminobenzenethiol on Ag nanoparticles. J. Phys. Chem. C 118, 6893–6902 (2014).
Zayak, A. et al. Chemical Raman enhancement of organic adsorbates on metal surfaces. Phys. Rev. Lett. 106, 083003 (2011).
Zhai, Y. et al. Polyvinylpyrrolidone-induced anisotropic growth of gold nanoprisms in plasmon-driven synthesis. Nat. Mater. 15, 889–895 (2016).
Gellé, A. et al. Applications of plasmon-enhanced nanocatalysis to organic transformations. Chem. Rev. 120, 986–1041 (2020).
Han, P. et al. Promoting Ni(II) catalysis with plasmonic antennas. Chem 5, 2879–2899 (2019).
**e, W. & Schlücker, S. Hot electron-induced reduction of small molecules on photorecycling metal surfaces. Nat. Commun. 6, 7570 (2015).
de Nijs, B. et al. Plasmonic tunnel junctions for single-molecule redox chemistry. Nat. Commun. 8, 994 (2017).
Wang, F. et al. Plasmonic harvesting of light energy for Suzuki coupling reactions. J. Am. Chem. Soc. 135, 5588–5601 (2013).
Marimuthu, A., Zhang, J. & Linic, S. Tuning selectivity in propylene epoxidation by plasmon mediated photo-switching of Cu oxidation state. Science 339, 1590–1593 (2013).
Huang, Y.-F. et al. Activation of oxygen on gold and silver nanoparticles assisted by surface plasmon resonances. Angew. Chem. Int. Ed. 53, 2353–2357 (2014).
Robatjazi, H. et al. Plasmon-induced selective carbon dioxide conversion on earth-abundant aluminum–cuprous oxide antenna–reactor nanoparticles. Nat. Commun. 8, 27 (2017).
Zhang, X. et al. Product selectivity in plasmonic photocatalysis for carbon dioxide hydrogenation. Nat. Commun. 8, 14542 (2017).
Hou, W. et al. Photocatalytic conversion of CO2 to hydrocarbon fuels via plasmon-enhanced absorption and metallic interband transitions. ACS Catal. 1, 929–936 (2011).
Yang, J., Guo, Y., Lu, W., Jiang, R. & Wang, J. Emerging applications of plasmons in driving CO2 reduction and N2 fixation. Adv. Mater. 30, 1802227 (2018).
Wang, S. et al. Positioning the water oxidation reaction sites in plasmonic photocatalysts. J. Am. Chem. Soc. 139, 11771–11778 (2017).
Devasia, D., Wilson, A. J., Heo, J., Mohan, V. & Jain, P. K. A rich catalog of C–C bonded species formed in CO2 reduction on a plasmonic photocatalyst. Nat. Commun. 12, 2612 (2021).
Zhou, L. et al. Light-driven methane dry reforming with single atomic site antenna-reactor plasmonic photocatalysts. Nat. Energy. 5, 61–70 (2020).
Yang, H. et al. Tunable wavelength enhanced photoelectrochemical cells from surface plasmon resonance. J. Am. Chem. Soc. 138, 16204–16207 (2016).
Oshikiri, T., Ueno, K. & Misawa, H. Plasmon-induced ammonia synthesis through nitrogen photofixation with visible light irradiation. Angew. Chem. Int. Ed. 53, 9802–9805 (2014).
Cortés, E. et al. Plasmonic hot electron transport drives nano-localized chemistry. Nat. Commun. 8, 14880 (2017).
Oksenberg, E. et al. Energy-resolved plasmonic chemistry in individual nanoreactors. Nat. Nanotech. 16, 1378–1385 (2021).
Sun, M. & Xu, H. A novel application of plasmonics: plasmon-driven surface-catalyzed reactions. Small 8, 2777–2786 (2012).
Grirrane, A., Corma, A. & García, H. Gold-catalyzed synthesis of aromatic azo compounds from anilines and nitroaromatics. Science 322, 1661–1664 (2008).
Wu, D.-Y. et al. Photon-driven charge transfer and photocatalysis of p-aminothiophenol in metal nanogaps: a DFT study of SERS. Chem. Commun. 47, 2520–2522 (2011).
Zhao, L.-B. et al. Theoretical study of plasmon-enhanced surface catalytic coupling reactions of aromatic amines and nitro compounds. J. Phys. Chem. Lett. 5, 1259–1266 (2014).
Dong, B., Fang, Y., Chen, X., Xu, H. & Sun, M. Substrate-, wavelength-, and time-dependent plasmon-assisted surface catalysis reaction of 4-nitrobenzenethiol dimerizing to p,p′-dimercaptoazobenzene on Au, Ag, and Cu films. Langmuir 27, 10677–10682 (2011).
**ao, Q. et al. Alloying gold with copper makes for a highly selective visible-light photocatalyst for the reduction of nitroaromatics to anilines. ACS Catal. 6, 1744–1753 (2016).
da Silva, A. G. et al. Plasmonic nanorattles as next‐generation catalysts for surface plasmon resonance‐mediated oxidations promoted by activated oxygen. Angew. Chem. Int. Ed. 55, 7111–7115 (2016).
Zong, Y. et al. Plasmon-induced decarboxylation of mercaptobenzoic acid on nanoparticle film monitored by surface-enhanced Raman spectroscopy. RSC Adv. 4, 31810–31816 (2014).
Wei, H., Loeb, S. K., Halas, N. J. & Kim, J.-H. Plasmon-enabled degradation of organic micropollutants in water by visible-light illumination of Janus gold nanorods. Proc. Natl Acad. Sci. USA 117, 15473–15481 (2020).
Devasenathipathy, R. et al. Plasmonic photoelectrochemical coupling reactions of para-aminobenzoic acid on nanostructured gold electrodes. J. Am. Chem. Soc. 144, 3821–3832 (2022).
Hao, C.-H. et al. Visible-light-driven selective photocatalytic hydrogenation of cinnamaldehyde over Au/SiC catalysts. J. Am. Chem. Soc. 138, 9361–9364 (2016).
Robatjazi, H. et al. Plasmon-driven carbon–fluorine (C(sp3)–F) bond activation with mechanistic insights into hot-carrier-mediated pathways. Nat. Catal. 3, 564–573 (2020).
Hung, W. H., Aykol, M., Valley, D., Hou, W. & Cronin, S. B. Plasmon resonant enhancement of carbon monoxide catalysis. Nano Lett. 10, 1314–1318 (2010).
Xu, G. et al. Unraveling the mechanism of ammonia selective catalytic oxidation on Ag/Al2O3 catalysts by operando spectroscopy. ACS Catal. 11, 5506–5516 (2021).
Tanaka, A., Hashimoto, K. & Kominami, H. Preparation of Au/CeO2 exhibiting strong surface plasmon resonance effective for selective or chemoselective oxidation of alcohols to aldehydes or ketones in aqueous suspensions under irradiation by green light. J. Am. Chem. Soc. 134, 14526–14533 (2012).
Li, H. et al. New reaction pathway induced by plasmon for selective benzyl alcohol oxidation on BiOCl possessing oxygen vacancies. J. Am. Chem. Soc. 139, 3513–3521 (2017).
Mukherjee, S. et al. Hot-electron-induced dissociation of H-2 on gold nanoparticles supported on SiO2. J. Am. Chem. Soc. 136, 64–67 (2014).
Quiroz, J. et al. Controlling reaction selectivity over hybrid plasmonic nanocatalysts. Nano Lett. 18, 7289–7297 (2018).
Mao, C. et al. Beyond the thermal equilibrium limit of ammonia synthesis with dual temperature zone catalyst powered by solar light. Chem 5, 2702–2717 (2019).
Tang, J., Durrant, J. R. & Klug, D. R. Mechanism of photocatalytic water splitting in TiO2. Reaction of water with photoholes, importance of charge carrier dynamics, and evidence for four-hole chemistry. J. Am. Chem. Soc. 130, 13885–13891 (2008).
Osterloh, F. E. Photocatalysis versus photosynthesis: a sensitivity analysis of devices for solar energy conversion and chemical transformations. ACS Energy Lett. 2, 445–453 (2017).
Liu, G. et al. Promoting active species generation by plasmon-induced hot-electron excitation for efficient electrocatalytic oxygen evolution. J. Am. Chem. Soc. 138, 9128–9136 (2016).
Zhang, Z. et al. A nonmetal plasmonic Z-scheme photocatalyst with UV- to NIR-driven photocatalytic protons reduction. Adv. Mater. 29, 1606688 (2017).
Zheng, B. Y. et al. Distinguishing between plasmon-induced and photoexcited carriers in a device geometry. Nat. Commun. 6, 7797 (2015).
Schlücker, S. Surface-enhanced Raman spectroscopy: concepts and chemical applications. Angew. Chem. Int. Ed. 53, 4756–4795 (2014).
Zhan, C., Moskovits, M. & Tian, Z.-Q. Recent progress and prospects in plasmon-mediated chemical reaction. Matter 3, 42–56 (2020).
Baffou, G., Bordacchini, I., Baldi, A. & Quidant, R. Simple experimental procedures to distinguish photothermal from hot-carrier processes in plasmonics. Light Sci. Appl. 9, 108 (2020).
Yu, Y., Sundaresan, V. & Willets, K. A. Hot carriers versus thermal effects: resolving the enhancement mechanisms for plasmon-mediated photoelectrochemical reactions. J. Phys. Chem. C 122, 5040–5048 (2018).
Ou, W. et al. Thermal and nonthermal effects in plasmon-mediated electrochemistry at nanostructured Ag electrodes. Angew. Chem. Int. Ed. 59, 6790–6793 (2020).
Li, W. et al. Single-molecular catalysis identifying activation energy of the intermediate product and rate-limiting step in plasmonic photocatalysis. Nano Lett. 20, 2507–2513 (2020).
Wright, D. et al. Mechanistic study of an immobilized molecular electrocatalyst by in situ gap-plasmon-assisted spectro-electrochemistry. Nat. Catal. 4, 157–163 (2021).
Choi, H.-K. et al. Single-molecule surface-enhanced Raman scattering as a probe of single-molecule surface reactions: promises and current challenges. Acc. Chem. Res. 52, 3008–3017 (2019).
Timoshenko, J. et al. Steering the structure and selectivity of CO2 electroreduction catalysts by potential pulses. Nat. Catal. 5, 259–267 (2022).
Handoko, A. D., Wei, F., Jenndy, Yeo, B. S. & Seh, Z. W. Understanding heterogeneous electrocatalytic carbon dioxide reduction through operando techniques. Nat. Catal. 1, 922–934 (2018).
Shao, F. et al. In-situ nanospectroscopic imaging of plasmon-induced two-dimensional [4+4]-cycloaddition polymerization on Au(111). Nat. Commun. 12, 4557 (2021).
Chikkaraddy, R. et al. Single-molecule strong coupling at room temperature in plasmonic nanocavities. Nature 535, 127–130 (2016).
Zhan, C. et al. Single-molecule plasmonic optical trap**. Matter 3, 1350–1360 (2020).
Lee, J., Crampton, K. T., Tallarida, N. & Apkarian, V. A. Visualizing vibrational normal modes of a single molecule with atomically confined light. Nature 568, 78–82 (2019).
Zhang, R. et al. Chemical map** of a single molecule by plasmon-enhanced Raman scattering. Nature 498, 82–86 (2013).
Mahapatra, S., Schultz, J. F., Li, L., Zhang, X. & Jiang, N. Controlling localized plasmons via an atomistic approach: attainment of site-selective activation inside a single molecule. J. Am. Chem. Soc. 144, 2051–2055 (2022). This article demonstrates the site-selective activation inside a single molecule by surface plasmons.
Zhao, J., Xue, S., Ji, R., Li, B. & Li, J. Localized surface plasmon resonance for enhanced electrocatalysis. Chem. Soc. Rev. 50, 12070–12097 (2021).
Acknowledgements
This work was supported by the Ministry of Science and Technology of China (2015CB932300) and the National Natural Science Foundation of China (21727807, 21533006, 22032004, 91427304, 22002036 and 22272140). C.Z. thanks the Alexander von Humboldt Foundation (AvH) for support with an AvH postdoctoral research grant. The authors thank B. Roldan (Fritz-Haber Institute of the Max-Planck Society) for helpful discussions.
Author information
Authors and Affiliations
Contributions
Introduction (C.Z., S.H., Z.-Q.T.); Experimentation (S.H., J.Y., C.Z.); Results (C.Z., X.-G.Z. D.-Y.W.); Applications (C.Z., Z.-Q.T.); Reproducibility and data deposition (C.Z.); Limitations and optimizations (C.Z., Z.-Q.T.); Outlook (all authors); Overview of the Primer (Z.-Q.T., C.Z.).
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Methods Primers thanks Andrea Baldi and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Direct charge transfer
-
The decay of a surface plasmon results in the direct excitation of an electron/hole into an unoccupied adsorbate orbital/semiconductor band with matching energy and leaves the hole/electron in the plasmonic nanostructure.
- Fermi–Dirac distribution
-
The distribution of particles over energy states in systems consisting of many identical particles that obey the Pauli exclusion principle.
- Hot carriers
-
Carriers including electrons or holes not in thermal equilibrium with the atoms in a material are frequently referred to as hot carriers.
- Indirect charge transfer
-
Excited carriers are formed in the plasmonic nanostructure with a roughly flat energy distribution and, subsequently, transfer to adsorbed molecules/semiconductor forming the charged state.
- Landau dam**
-
A process occurring because of the energy exchange between an electromagnetic wave and particles in the plasma, which can interact strongly with the wave. In a surface plasmon system, the Landau dam** process results in direct transition between two states near the Fermi surface, assisted by the surface plasmon momentum, and creating a hot hole and a hot electron.
- Lossy media
-
In optics, the media are not transparent to the incident light waves and result in absorptions. The dissipation of a medium is usually described by the imaginary part of its dielectric permittivity.
- Periodic boundary condition
-
A term often used in computer simulations and mathematical models, for which a unit cell is chosen to approximate a large (infinite) system.
- Photothermal effect
-
The conversion of photon energy to thermal energy.
- Surface plasmon resonance
-
(SPR). The resonant collective oscillation of conduction electrons in nanostructures stimulated by incident light.
- Turnover frequency
-
The derivative of the number of turnovers with respect to the time per active site.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhan, C., Yi, J., Hu, S. et al. Plasmon-mediated chemical reactions. Nat Rev Methods Primers 3, 12 (2023). https://doi.org/10.1038/s43586-023-00195-1
Accepted:
Published:
DOI: https://doi.org/10.1038/s43586-023-00195-1
- Springer Nature Limited
This article is cited by
-
Photocatalytic CO2 reduction
Nature Reviews Methods Primers (2023)
-
Surface Plasmon Excitation: Theory, Configurations, and Applications
Plasmonics (2023)