Abstract
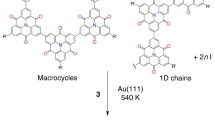
Creating conjugated macrocycles has attracted extensive research interest because their unique chemical and physical properties, such as conformational flexibility, intrinsic inner cavities and aromaticity/antiaromaticity, make these systems appealing building blocks for functional supramolecular materials. Here, we report the synthesis of four-, six- and eight-membered tetraphenylethylene (TPE)-based macrocycles on Ag(111) via on-surface Ullmann coupling reactions. The as-synthesized macrocycles are spontaneously segregated on the surface and self-assemble as large-area two-dimensional mono-component supramolecular crystals, as characterized by scanning tunneling microscopy (STM). We propose that the synthesis benefits from the conformational flexibility of the TPE backbone in distinctive multi-step reaction pathways. This study opens up opportunities for exploring the photophysical properties of TPE-based macrocycles.
Similar content being viewed by others
Introduction
Since the discovery of macrocyclic crown-ethers by Pedersen more than half a century ago1, macrocycles have become versatile building blocks in supramolecular chemistry2. Specifically, inner cavities of shape-persistent macrocycles can host molecules or ions forming host-guest supramolecular complexes3,4. In contrast to the open-chain oligomers, the cyclic topology gives rise to unique electronic and optical properties such as aromaticity/antiaromaticity5, collective spin excitations6,7, enhanced nonlinear optical responses8, and acting as molecular quantum rings9. Macrocycles with extended conjugation have attracted extensive attentions for their potential applications in organic solar cells, photodetectors, organic light-emitting diodes, drug discovery, and many others2,10,11,12. Moreover, owing to their structural rigidity and adaptivity, macrocycles can be assembled into one-dimensional tubular structures13, lamellar assemblies14, two-dimensional (2D) and three-dimensional (3D) organic crystals, which exhibit emerging properties such as semiconducting, porosity, etc.14,15,16.
As an efficient method to fabricate organic nanostructures with atomic precision17,18,19, on-surface synthesis has been used to synthesize poly-phenyl macrocycles in recent years20,21,22,23,24,25. The underlying premise of this bottom-up strategy is that precursor molecules undergo a well-defined sequence of inter- and intramolecular reactions, usually leading to the formation of one kind of macrocycle as the predominant product. In this work, we apply on-surface coupling reactions to synthesize a family of macrocycles using a precursor of tetraphenylethylene (TPE) derivative, 4,4′-(2,2-diphenylethene-1,1-diyl)bis(bromobenzene) (Br2-TPE). TPE is a prototype aggregation-induced emission (AIE) chromophore26,27. Macrocycles made of TPE moieties linked with saturated bonds exhibit excellent fluorescence performance and improved selectivity and sensitivity in sensors due to the enhanced AIE effect4e presents a schematic model of a unit cell, showing the neighboring M8 macrocycles interact with each other via similar π–π interactions between the approaching exterior phenyl groups (ring-to-ring distance: 0.53 ± 0.04 nm).
a Large-scale STM image (200 nm × 120 nm; −2.0 V, 20 pA) of the 2D crystalline monolayer made of eight-membered macrocycles (M8). Inset: FFT pattern. b Zoom in STM image (−1.0 V, 50 pA) of the M8 2D crystal with overlaid simplified molecular models. The blue square denotes the unit cell. c, d High-resolution STM image (−1.0 V, 150 pA) and chemical model of M8, outlined by the dashed white line. Scale bar: 1 nm. e Schematic model of the M8 2D crystal.
Besides the three even-membered macrocycles, the on-surface coupling reaction also yields macrocycles consisting of five (M5) and seven (M7) TPE units, and open-end oligomer chains of different length. Supplementary Fig. 1 shows these structures are mixed on the substrate forming disordered monolayer. In comparison, the M6, M4 and M8 macrocycles are the predominant products and can assemble as the mono-component supramolecular 2D crystals with very large size. It is worth mentioning that the large-scale 2D crystalline islands made of M6, M4 and M8 macrocycles (shown in Figs. 2a, 3a, and 4a) are observed in different areas of the same sample. Therefore, the on-surface synthesized macrocycles are spontaneously segregated into mono-component domains. The M6 2D crystals can extend to micrometer size and cover nearly entire terraces, as shown in Supplementary Fig. 4. The 2D crystals fail to grow crossing the steps, indicating that only the width of the Ag(111) terrace limits the size of the 2D crystals. The M4 and M8 2D crystals are not as big as those of M6, nevertheless, can extend over ~20,000 nm2, as shown in Figs. 3a and 4a. Note that we have not observed boundaries between the mono-component supramolecular islands even in the micrometer scale images.
Multi-step reaction pathways
Here we discuss the possible reaction pathways of M4, M6 and M8. As illustrated in Fig. 5, we propose a multi-step ring formation mechanism: the first step is coupling of two TPE monomers forming a dimer (BTPE), as represented with the green links. The dimer can be a cis-conformation or a trans-conformation. Next, coupling of two (three) cis-BTPEs, as represented with the red links, results in an M4 (M6) ring. cis-BTPEs can be coupled to form open-end oligomer chains too. Coupling of trans-BTPEs always forms open-end oligomer chains. Interestingly, cross-coupling of a cis-BTPE and a trans-BTPE forms an intermediate, further coupling of two intermediates, as represented with the orange links, may yield an M8 macrocycle with a Cassini oval shape. The products of M5 or M7 can be formed by ring-closing coupling of one TPE unit with two or three cis-BTPEs (Supplementary Fig. 7), respectively.
To date, it has been reported that on-surface synthesis always yields only one predominant macrocycle product20,21,23. For example, a precursor with meta-linkage yields hexagonal macrocycles. We attribute the multiple products in our work to the structural flexibility of the TPE backbone thanks to its non-planar conformation. The DFT optimized structure of a Br2-TPE precursor reveals that the opening angle between the two bromobenzene groups (denoted as ph-ethy-ph angle) is ~114.65°, as shown in Supplementary Fig. 8a, which favors hexagonal shaped M6. In contrast, the M4 and M8 macrocycles contain the TPE units with ph-ethy-ph angles deviated from 120° owing to the deformed TPE backbones. Note that recent optical studies demonstrate that the emission behaviors of TPE units vary against conformation changes35,36. Therefore, the M4, M6, and M8 macrocycles, which consist of TPE units with different conformations, provide a platform to explore conformation-dependent photophysical properties.
Discussion
The growth of such large-area mono-component 2D supramolecular crystals is very rare in a multi-component system. We propose that in these mono-component 2D crystals the periphery phenyl groups of neighboring macrocycles are maximally inter-connected, specifically, the hexagonal M6 in the three-fold packing, the square M4 in the four-fold packing, and the Cassini oval shape M8 in the basketweave packing, so that the mono-component 2D crystals are energetically favored. The assembly process, which takes place at 200 °C, is highly dynamic, involving spontaneous segregation and self-assembly of the as-formed macrocycles on the surface. The segregation requires mutual recognition of different-type macrocycles, while the self-assembly requires self-recognition of same-type macrocycles. Without direct experimental observation or sophisticated theoretical simulation, this intricate process is beyond our comprehension. We propose two possible growth modes: (1) the macrocycles are randomly mixed at 200 °C and undergo phase separation in the cooling process, resulting in the mono-component supramolecular islands; (2) segregation and self-assembly of the macrocycles occur at 200 °C. Since we do not have direct experimental data revealing the high-temperature process, we cannot draw a conclusion which one is true. Nevertheless, the huge size of the mono-component islands and absence of domain boundaries imply that the segregation and self-assembly are high effective, so we incline to the latter scenario.
In summary, we demonstrate “one-pot” synthesis of 4-, 6-, and 8-membered TPE macrocycles on a Ag(111) surface as the predominant products, benefiting from the structural flexibility of TPE backbone. These macrocycles are spontaneously segregated on the surface and self-assemble as mono-component 2D supramolecular crystals with very large areas. Our findings provide a design strategy toward precise synthesis of multi-component macrocycles on a surface. We foresee that the large-area synthesis of shape-persistent conjugated oligo-TPE macrocycles will enable exploration of their photophysical properties using STM-induced luminescence37,38,39.
Methods
Sample preparation and STM measurements
All experiments were carried out in an ultrahigh vacuum system (base pressure <3.0 × 10−10 mbar) equipped with a CreaTec low-temperature STM. A single-crystalline Ag(111) substrate was cleaned via repeated cycles of Ar+ sputtering (0.8 keV) and subsequent annealing at 450 °C. Molecules of 4,4′-(2,2-diphenylethene-1,1-diyl)bis(bromobenzene) were sublimated from a Knudsen cell at 140 °C, to achieve a deposition rate of ~0.66 ML/h, while the substrate was kept at 200 °C during molecule deposition. A monolayer is defined as the amount of deposited molecules that entirely covers the substrate surface. The slow deposition on a hot surface was used to promote the yield of macrocycles by achieving pseudo-high-dilution31. After growth, the sample was transferred to STM chambers for STM measurements. The STM images were taken at either 77 K or 5.3 K in the constant-current mode. All the lateral distances are measured from the STM images at 5.3 K.
Theoretical calculations
All DFT calculations were performed by using Gaussian 09 program40 using the B3LYP method with the 6–31G(d,p) basis set for structure optimization. Molecular geometries are generated by IQmol molecular viewer.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Pedersen, C. J. The discovery of crown ethers (noble lecture). Angew. Chem. Int. Ed. 27, 1021–1027 (1988).
Iyoda, M., Yamakawa, J. & Rahman, M. J. Conjugated macrocycles: concepts and applications. Angew. Chem. Int. Ed. 50, 10522–10553 (2011).
Shimizu, H. et al. Synthesis, structures, and photophysical properties of π-expanded oligothiophene 8-mers and their saturn-like C60 complexes. J. Am. Chem. Soc. 137, 3877–3885 (2015).
Bols, P. S. & Anderson, H. L. Template-directed synthesis of molecular nanorings and cages. Acc. Chem. Res. 51, 2083–2092 (2018).
Peeks, M. D., Claridge, T. D. W. & Anderson, H. L. Aromatic and antiaromatic ring currents in a molecular nanoring. Nature 541, 200–203 (2017).
Hieulle, J. et al. On‐surface synthesis and collective spin excitations of a triangulene‐based nanostar. Angew. Chem. Int. Ed. 60, 25224–25229 (2021).
Mishra, S. et al. Observation of fractional edge excitations in nanographene spin chains. Nature 598, 287–292 (2021).
Williams-Harry, M. et al. Giant thienylene-acetylene-ethylene macrocycles with large two-photon absorption cross section and semishape-persistence. J. Am. Chem. Soc. 130, 3252–3253 (2008).
Judd, C. J. et al. Molecular quantum rings formed from a π-conjugated macrocycle. Phys. Rev. Lett. 125, 206803 (2020).
Ball, M. et al. Conjugated macrocycles in organic electronics. Acc. Chem. Res. 52, 1068–1078 (2019).
Marsault, E. & Peterson, M. L. Macrocycles are great cycles: applications, opportunities, and challenges of synthetic macrocycles in drug discovery. J. Med. Chem. 54, 1961–2004 (2011).
Iyoda, M. & Shimizu, H. Multifunctional π-expanded oligothiophene macrocycles. Chem. Soc. Rev. 44, 6411–6424 (2015).
De Greef, T. F. A. et al. Supramolecular polymerization. Chem. Rev. 109, 5687–5754 (2009).
Kudernac, T., Lei, S., Elemans, J. A. A. W. & De Feyter, S. Two-dimensional supramolecular self-assembly: nanoporous networks on surfaces. Chem. Soc. Rev. 38, 402–421 (2009).
Iyoda, M. et al. Multifunctional π-expanded macrocyclic oligothiophene 6-Mers and related macrocyclic oligomers. J. Am. Chem. Soc. 136, 2389–2396 (2014).
Zhou, Y., Jie, K., Zhao, R. & Huang, F. Supramolecular-macrocycle-based crystalline organic materials. Adv. Mater. 32, 1904824 (2020).
Dong, L., Liu, P. N. & Lin, N. Surface-activated coupling reactions confined on a surface. Acc. Chem. Res. 48, 2765–2774 (2015).
Grill, L. & Hecht, S. Covalent on-surface polymerization. Nat. Chem. 12, 115–130 (2020).
Clair, S. & de Oteyza, D. G. Controlling a chemical coupling reaction on a surface: tools and strategies for on-surface synthesis. Chem. Rev. 119, 4717–4776 (2019).
Chen, M. et al. On-surface synthesis and characterization of honeycombene oligophenylene macrocycles. ACS Nano 11, 134–143 (2017).
Fan, Q. et al. Surface‐assisted organic synthesis of hyperbenzene nanotroughs. Angew. Chem. Int. Ed. 52, 4668–4672 (2013).
Lipton‐Duffin, J. A., Ivasenko, O., Perepichka, D. F. & Rosei, F. Synthesis of polyphenylene molecular wires by surface‐confined polymerization. Small 5, 592–597 (2009).
Steiner, C. et al. Hierarchical on-surface synthesis and electronic structure of carbonyl-functionalized one- and two-dimensional covalent nanoarchitectures. Nat. Commun. 8, 14765 (2017).
Liu, M. et al. High-yield formation of graphdiyne macrocycles through on-surface assembling and coupling reaction. ACS Nano 12, 12612–12618 (2018).
Fan, C. et al. On-surface synthesis of giant conjugated macrocycles. Angew. Chem. Int. Ed. 60, 13896–13899 (2021).
Mei, J., Leung, N. L. C., Kwok, R. T. K., Lam, J. W. Y. & Tang, B. Z. Aggregation-induced emission: together we shine, united we soar! Chem. Rev. 115, 11718–11940 (2015).
Zhao, Z., Lam, J. W. Y. & Tang, B. Z. Tetraphenylethene: a versatile AIE building block for the construction of efficient luminescent materials for organic light-emitting diodes. J. Mater. Chem. 22, 23726–23740 (2012).
Feng, H.-T., Yuan, Y.-X., **ong, J.-B., Zheng, Y.-S. & Tang, B. Z. Macrocycles and cages based on tetraphenylethylene with aggregation-induced emission effect. Chem. Soc. Rev. 47, 7452–7476 (2018).
Liu, Y. et al. Shape-persistent π-conjugated macrocycles with aggregation-induced emission property: synthesis, mechanofluorochromism, and mercury(II) detection. ACS Appl. Mater. Interfaces 11, 34232–34240 (2019).
Lei, S. N. et al. BowtieArene: a dual macrocycle exhibiting stimuli‐responsive fluorescence. Angew. Chem. Int. Ed. 59, 10059–10065 (2020).
Fan, Q. et al. On-surface pseudo-high-dilution synthesis of macrocycles: principle and mechanism. ACS Nano 11, 5070–5079 (2017).
Eichhorn, J. et al. On-surface Ullmann polymerization via intermediate organometallic networks on Ag(111). Chem. Commun. 50, 7680–7682 (2014).
Sinnokrot, M. O., Valeev, E. F. & Sherrill, C. D. Estimates of the ab initio limit for π−π interactions: the benzene dimer. J. Am. Chem. Soc. 124, 10887–10893 (2002).
Lee, E. C. et al. Understanding of assembly phenomena by aromatic−aromatic interactions: benzene dimer and the substituted systems. J. Phys. Chem. A 111, 3446–3457 (2007).
Guo, Z. et al. Drum-like metallacages with size-dependent fluorescence: exploring the photophysics of tetraphenylethylene under locked conformations. J. Am. Chem. Soc. 143, 9215–9221 (2021).
Shi, Y. et al. Multiple yet switchable hydrogen-bonded organic frameworks with white-light emission. Nat. Commun. 13, 1882 (2022).
Rossel, F., Pivetta, M. & Schneider, W.-D. Luminescence experiments on supported molecules with the scanning tunneling microscope. Surf. Sci. Rep. 65, 129–144 (2010).
Qiu, X., Nazin, G. & Ho, W. Vibrationally resolved fluorescence excited with submolecular precision. Science 299, 542–546 (2003).
Zhang, L. et al. Electrically driven single-photon emission from an isolated single molecule. Nat. Commun. 8, 580 (2017).
Frisch, M. J. et al. Gaussian 09, Revision D.01 (Gaussian, Inc., 2013).
Acknowledgements
This work is financially supported by the Research Grants Council of Hong Kong (C6014-20W and 16301219) and the Innovation and Technology Commission (ITC-CNERC14SC01).
Author information
Authors and Affiliations
Contributions
N.L. conceived the project; E.L., C.K.L., and C.C. performed the on-surface synthesis and STM experiments; H.X. synthesized the precursor molecules under the supervision of J.W.Y.L. and B.Z.T.; E.L. performed DFT calculations with assistance from J.Z.; the manuscript was written by E.L. and N.L. with contributions from all co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, E., Lyu, CK., Chen, C. et al. On-surface synthesis and spontaneous segregation of conjugated tetraphenylethylene macrocycles. Commun Chem 5, 174 (2022). https://doi.org/10.1038/s42004-022-00794-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-022-00794-1
- Springer Nature Limited