Abstract
Ketones are crucial intermediates in synthesis and frequent moieties in many products. The direct regioselective synthesis of ketones from internal alkenes could simplify synthetic routes and solve a long-standing challenge in catalysis. Here we report the laboratory evolution of a cytochrome P450 enzyme for the direct oxidation of internal arylalkenes to ketones with several thousand turnovers. This evolved ketone synthase benefits from 15 crucial mutations, most of them distal to the active site. Computational analysis revealed that all these mutations collaborate to generate and tame a highly reactive carbocation intermediate. This is achieved through a confined, rigid, and geometrically and electrostatically preorganized active site. The engineered enzyme exploits a metal–oxo species for ketone synthesis and enables various challenging alkene functionalization reactions. This includes the catalytic, enantioselective oxidation of internal alkenes to ketones and formal asymmetric hydrofunctionalizations of internal alkenes in combination with other biocatalysts.

Similar content being viewed by others
Main
Ketones are essential structural motifs in pharmaceuticals, agrochemicals, materials and natural products. They are also versatile reactants in a wide range of reactions, for example, in the synthesis of chiral alcohols and amines1. Thus, efficient catalytic methods to produce ketones have long been sought. Selective ketone synthesis via the simple aerobic oxidation of internal alkenes would represent a powerful addition to synthetic organic chemistry. This is because internal alkenes are easily accessible from petroleum and renewable resources as well as from well-established reactions such as olefin metathesis2 and carbonyl olefination3. Due to the lack of efficient and selective catalysts, the hydroboration–oxidation method using toxic and expensive boranes is still widely used4,5. In addition to methods using stoichiometric reagents, various catalytic strategies to access ketones from internal alkenes have been developed. In particular, attempts have been made to expand the well-studied Wacker–Tsuji oxidation from terminal to internal alkenes6,7,8,9. However, internal alkenes are less reactive in such palladium-catalysed Wacker-type oxidations, resulting in high catalyst loadings and consequently low total turnover numbers (<20). In addition to activity, selectivity is also a major challenge in catalyst development (Fig. 1a). High regioselectivities in Wacker-type oxidation reactions depend on specific internal alkenes bearing directing groups that favour the formation of one ketone over the other. Many of these protocols are not aerobic oxidations or depend on stoichiometric reagents such as peroxides and phenylsilanes10,11. A recently published dual catalytic approach used water as the terminal oxidant and produced hydrogen gas as a by-product12. In general, efficient and regioselective aerobic oxidation of internal alkenes to ketones is not only a considerable challenge in catalyst development, but is also highly desired as it has the potential to streamline the synthesis of many important molecules.
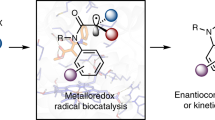
a, Regioselectivity is a challenge in the catalytic oxidation of internal alkenes to ketones. b, Design of a catalytic process to convert internal alkenes into ketones using high-valent metal–oxo species as the catalytic oxidant. High-valent metal–oxo species typically react with alkenes in epoxidation reactions. The ketone product is accessible by stabilizing a carbocation intermediate, and the reaction proceeds via a coupled electron–hydride transfer. c, Given that enzymatic alkene to ketone oxidation can be accessed, many challenging functionalization reactions of internal alkenes can be realized in combination with established biocatalysts such as ketoreductases39,40 (i), ω-transaminases39 (ii) and imine reductases41,42 (iii).
We have recently started to explore the potential of enzymes to directly oxidize alkenes to carbonyl compounds13,14. In an initial study, we applied directed evolution to engineer an enzyme that oxidizes terminal alkenes to the corresponding aldehydes, attaining opposite selectivity to the widely used Wacker–Tsuji oxidation reaction. The evolved iron haem-based biocatalyst achieved aldehyde formation by controlling an oxo transfer reaction using a high-valent metal–oxo complex as the catalytic oxidant (Fig. 1b). Many catalysts comprising such metal–oxo complexes, including cytochrome P450s15,16, peroxygenases17 and Jacobsen’s catalyst18,19, are used to efficiently epoxidize alkenes via highly concerted reaction pathways. The key to harnessing oxo transfer for carbonyl-selective alkene oxidation is to create a catalyst that is able to intercept the epoxidation and couple oxo transfer with an electron–hydride transfer process (Fig. 1b). This can be achieved by accessing a highly reactive carbocation intermediate and making it available for organic synthesis. While this is currently out of reach for small-molecule catalysts, our recent study13 showed that reactive centres in enzymes can be optimized with high precision to fully access and exploit such reactive carbocation intermediates. Recent mechanistic studies on this oxo transfer reaction revealed that alkene epoxidation is a dynamically favoured process and that carbonyl formation can be accomplished by controlling the accessible conformations of the radical and carbocation intermediates14. In addition, electrostatic preorganization of the enzyme active site favours the formation of this key carbocation intermediate14.
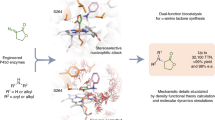
Here we report a generalizable approach to develop enzymes for the aerobic oxidation of alkenes to ketones. In particular, we have expanded the enzymatic alkene-to-carbonyl oxidation from terminal to internal arylalkenes. This generates ketones with high activity and regioselectivity in a direct aerobic oxidation reaction. Key aspects of this study are the directed enzyme evolution of a ketone synthase as well as computational studies to rationalize and propose how more than a dozen beneficial mutations collaborate to access this catalytic cycle. Furthermore, we applied the ketone synthase in synthesis as a stand-alone catalyst to generate various benzyl ketones as well as in combination with established biocatalysts in cascade reactions. The latter application enabled the formal asymmetric hydration and hydroamination (Fig. 1c) of internal alkenes, reactions that are particularly sought after with only limited catalytic solutions (Supplementary Figs. 2 and 3)20,21,22,23, SPMs33 calculated from accumulated MD simulation time for variants P7E and KS in their holo and ‘substrate bound in a NAC conformation’ states. The sizes of the spheres (nodes of the network) and black lines (edges of the network) are indicative of their weight in the network. Altered amino acid residues during evolution are shown in stick format and highlighted in orange. Haem cofactor and substrate 1 are shown in stick format, in grey and cyan, respectively. Insets: active site with bound substrate.
Substrate scope and application in synthesis
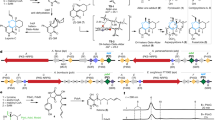
To explore the potential applications of such a KS, we studied the substrate scope of this enzyme and performed reactions on a preparative scale. The KS with its confined active site accepted various structurally related trans-β-methylstyrene derivatives and produced the corresponding ketones with efficiencies of up to several thousand TTNs and selectivities of up to 79% (Fig. 6a). Various substitutions are tolerated, including substitutions in the ortho (11), meta (10) and para (4–8) positions of the aromatic ring as well as in the trans-β-alkenic position (9). We also tested various structurally more unrelated internal alkenes, but these were not accepted as substrates by the highly optimized active site (Supplementary Fig. 10). As an example, cis-β-methylstyrene was converted by the KS into epoxides but not phenylacetone (Supplementary Fig. 11). This agrees with recent mechanistic findings14 on the evolved cytochrome P450 that oxidized terminal alkenes to aldehydes13, highlighting that hydride migration during the catalytic cycle of these evolved enzymes based on P450LA1 proceeds selectively from the cis position. This also supports our conclusions that catalysts optimized for metal–oxo-mediated alkene-to-ketone oxidation require a highly confined and preorganized reactive centre.
a, Substrate scope of the KS reaction. Reactions were carried out using 0.625 µM KS, 5 mM of the corresponding substrate and 5 mM NADH cofactor. TTN values were determined after 2 h reaction time. aMaximum TTN was determined after 48 h biotransformation combined with a cofactor regeneration system. b, KS application on a preparative scale for the synthesis of ketones as well as in the formal asymmetric hydration and hydroamination of internal alkenes. See Supplementary Figs. 16 and 17 for more details. e.r., enantiomeric ratio; GDH, glucose dehydrogenase; PAR, phenylacetaldehyde reductase; LBv-ADH, alcohol dehydrogenase from Lactobacillus brevis; IRED, imine reductase pIR-23 (Cystobacter ferrugineus (CF)IRED).
Next, we explored substitutions at the α-position to study the enantioselectivity of the enzymatic oxidation reaction. Even though trans-α,β-dimethylstyrene (17) was not used as substrate in the screening, and the enzyme therefore was not selected for enantioselectivity during the directed evolution, the KS enabled the synthesis of the chiral ketone 12 with good enantioselectivity towards the (S) enantiomer (enantiomeric ratio, 87:13, S/R; Fig. 6a and Supplementary Fig. 13). Stereoselectivity is achieved by enantiofacial discrimination during the 1,2-hydride migration after the first C–O bond formation, in line with previous observations for a related engineered enzyme14. The enantioselectivity detected is also consistent with the selectivity model proposed on the basis of the computational modelling carried out on trans-β-methylstyrene (Supplementary Fig. 13). It is worth highlighting that catalytic asymmetric oxidation of internal alkenes to chiral ketones is currently unknown. Further engineering of this cytochrome P450 enzyme or other iron-dependent monooxygenases will reveal whether KSs can be generated with even higher enantioselectivity.
The activity of the KS could be further optimized by changing the reaction conditions. Lengthening the reaction time and application of a cofactor recycling system enabled the conversion of alkene 1 to ketone 2 with a TTN of up to 4,750 (Supplementary Fig. 14). To demonstrate that these reactions can be performed on a preparative scale (1.0 mmol), phenylacetone (2) was synthesized using a catalyst loading of 0.025 mol% KS (Fig. 6b). The product was isolated in 61% yield, with atmospheric oxygen and glucose as the only stoichiometric reagents.
Formal asymmetric hydrofunctionalization of internal alkenes
Laboratory evolution of non-natural enzyme function can provide access to new synthetic pathways and solve long-standing challenges in synthesis36,37,38. In this regard, we performed asymmetric redox hydrations and hydroaminations on internal arylalkenes by combining the KS with ketoreductases39,40 and imine reductases41,42 in cascade reactions on a preparative scale (1.0 mmol). Using this set-up, the unactivated internal arylalkene 1 was converted into chiral phenylethanols and phenylethylamine, which are important structural motifs in top-selling pharmaceuticals (Supplementary Fig. 15). The chiral alcohols 13 and 14 as well as amine 16 were produced with excellent enantioselectivity (enantiomeric ratio of up to >99:1) in isolated yields of 66, 69 and 39%, respectively (Fig. 6b). An important feature of these reactions is that (1) they only depend on simple stoichiometric reagents such as atmospheric oxygen and isopropanol (asymmetric hydration) or atmospheric oxygen and glucose (asymmetric hydroamination) and (2) simple unprotected amines (such as 15) can be used as amine donors. This approach can in principle be expanded to other internal arylalkenes (Fig. 6a) or other amines as amine donors42,43.
In addition, we aimed to generate two stereocentres using the trisubstituted alkene 17 as the starting material (Fig. 7). Alcohol 18 was obtained via ketone 12 with low activity but excellent selectivity, yielding a single stereoisomer. This approach combines the KS-catalysed enantioselective alkene-to-ketone oxidation via an asymmetric hydride migration with diastereofacial differentiation of the ketoreductase.
Alkene 17 was used as a substrate to generate two stereocentres. The enzymatic reaction generates a single stereoisomer of alcohol 18 via ketone 12 and combines enantioselective oxidation by the KS with diastereofacial discrimination by a ketoreductase (PAR). The enzymatic reaction is compared with a chemical synthesis that generates all possible stereoisomers (see Supplementary Fig. 18 for more information).
Such conversions of unactivated internal alkenes to chiral alcohols and amines are a particular challenge in catalysis (Supplementary Figs. 2 and 3) with only limited catalytic solutions20,21,22,23,63.
MD simulations
MD simulations in explicit water were performed using the AMBER18 package64,65. Parameters for the trans-β-methylstyrene (1) substrate were generated within the Antechamber66 module in the AMBER18 package using the general AMBER force field (gaff2) with partial charges set to fit the electrostatic potential generated at the HF/6-31G(d) level by the restrained electrostatic potential model. Parameters for the haem compound I and the axial Cys were taken from ref. 67. The enzyme variants were solvated in a pre-equilibrated cubic box with a 10-Å buffer of transferable intermolecular potential water molecules using the AMBER18 leap module, resulting in the addition of ∼16,000 solvent molecules. Explicit counter ions (Na+ or Cl−) were introduced to neutralize the system. All subsequent calculations were performed using the Stony Brook modification of the Amber14 force field (ff14SB)68. A two-stage geometry optimization approach was used. In the first stage, the positions of solvent molecules and ions were minimized by imposing positional restraints on the solute by a harmonic potential with a force constant of 500 kcal mol−1 Å−2, and the second stage involved an unrestrained minimization of all the atoms in the simulation cell. The system was gently heated in six 50 ps steps, increasing the temperature by 50 K in each step (0–300 K) under constant volume and periodic boundary conditions. Water molecules were treated using the SHAKE algorithm, kee** the angle between the hydrogen atoms fixed. Long-range electrostatic effects were modelled using the particle-mesh Ewald method. An 8-Å cut-off was applied to Lennard-Jones and electrostatic interactions. Harmonic restraints of 30 kcal mol–1 were applied to the solute, and the Langevin scheme was used to control and equalize the temperature. The time step was kept at 1 fs during the heating stages. Each system was then equilibrated for 2 ns with a 2 fs time step at a constant pressure of 1 atm and temperature of 300 K without restraints. Once the systems had been equilibrated in the constant-temperature and constant-pressure ensemble, production trajectories were run under the constant-temperature and constant-volume ensemble and periodic boundary conditions. In particular, a total of 5,000 ns of simulations in the absence of substrate were accumulated for variants P7E and KS from five independent replicas of 1,000 ns for each system. The Cpptraj module from Ambertools utilities was used to process and analyse the trajectories, including cluster analyses. POVME 3.0 was used to analyse active-site volumes69.
Docking and substrate-bound MD simulations
The most representative structures from the previous holo state simulations were characterized by clustering of the accumulated simulation time, considering the protein backbone root mean square deviation. These structures were used for docking calculations with substrate 1, which were performed using AutoDock Vina70. The docking results were used as starting points for the restrained MD simulations, in which the distance between the centre of mass of the alkene (defined by atoms C1 and C2) of substrate 1 and the oxygen atom of haem compound I was kept restrained during the MD simulation (3.0–3.5 Å, using a 100 kcal mol−1 Å−2 force constant). This allowed the catalytically relevant binding poses of the substrate to be explored when it is in a NAC conformation to make the oxidation reaction happen, largely refining the docking predictions and preventing undesired unbinding events during the simulations. The same protocol previously described for the MD simulations was applied. A total of five independent replicas of 500 ns of production trajectory each were accumulated for each system, accumulating a total of 2.5 µs of restrained MD simulation time for each system. Substrate–residue interactions were calculated using the pairwise per-residue free-energy decomposition and molecular mechanics with generalized Born and surface area solvation (MM-GBSA) approach as implemented in the MMPBSA.py module71 from AmberTools18. The MM-GBSA energies of 500 structures (1 ns each) were calculated for each MD trajectory. The final reported MM-GBSA energy was the average of the 500 structures. SPMs for dynamic correlation network analysis were estimated using the module DynaComm.py (ref. 33).
QM/MM calculations
Initial structures for QM/MM modelling were selected from the substrate-bound restrained MD simulations of the KS. Representative snapshots were selected on the basis of the different binding poses explored by the substrate. All water molecules and counter ions beyond 3 Å from any residue of the protein, cofactors or substrates were removed. The QM region included the haem porphyrin pyrrole core, the Cys390 side chain, the iron centre and the whole substrate (1). The resulting QM region had a neutral charge, and both doublet and quartet low-lying electronic states were considered. All residues and water molecules outside a 12-Å shell from the QM region were kept frozen. QM/MM calculations were carried out using the ONIOM72 approach as implemented in the Gaussian 09 package58. Geometry optimizations were performed with the hybrid (U)B3LYP functional59,60,61 using an ultrafine integration grid and the 6-31G(d) basis set on all atoms except for iron, for which an SDD basis set and related SDD pseudopotential were used. The MM parameters and charges were identical to those used in the MD simulations. A two-step sequential optimization protocol using the QuadMacro optimization algorithm was used: a first optimization using a mechanical embedding scheme was initially performed, and once optimized, MM water molecules were kept frozen and a second optimization was performed within the electrostatic embedding scheme. Stationary points were verified as minima or saddle-point (transition-state) geometries after vibrational frequency analysis, and thermal corrections were obtained at 1 atm and 298.15 K. Single-point energy calculations on the optimized structures were performed at the (U)B3LYP/Def2TZVP level of theory within the electrostatic embedding scheme, and included empirical Grimme D3 dispersion corrections with Becke–Johnson (D3BJ) dam**63.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.