Abstract
Cerebrospinal fluid tumor-derived DNA (CSF-tDNA) analysis is a promising approach for monitoring the neoplastic processes of the central nervous system. We applied a lung cancer-specific sequencing panel (CAPP-Seq) to 81 CSF, blood, and tissue samples from 24 lung cancer patients who underwent lumbar puncture (LP) for suspected leptomeningeal disease (LMD). A subset of the cohort (N = 12) participated in a prospective trial of osimertinib for refractory LMD in which serial LPs were performed before and during treatment. CSF-tDNA variant allele fractions (VAFs) were significantly higher than plasma circulating tumor DNA (ctDNA) VAFs (median CSF-tDNA, 32.7%; median plasma ctDNA, 1.8%; P < 0.0001). Concentrations of tumor DNA in CSF and plasma were positively correlated (Spearman’s ρ, 0.45; P = 0.03). For LMD diagnosis, cytology was 81.8% sensitive and CSF-tDNA was 91.7% sensitive. CSF-tDNA was also strongly prognostic for overall survival (HR = 7.1; P = 0.02). Among patients with progression on targeted therapy, resistance mutations, such as EGFR T790M and MET amplification, were common in peripheral blood but were rare in time-matched CSF, indicating differences in resistance mechanisms based on the anatomic compartment. In the osimertinib cohort, patients with CNS progression had increased CSF-tDNA VAFs at follow-up LP. Post-osimertinib CSF-tDNA VAF was strongly prognostic for CNS progression (HR = 6.2, P = 0.009). Detection of CSF-tDNA in lung cancer patients with suspected LMD is feasible and may have clinical utility. CSF-tDNA improves the sensitivity of LMD diagnosis, enables improved prognostication, and drives therapeutic strategies that account for spatial heterogeneity in resistance mechanisms.
Similar content being viewed by others
Introduction
Metastasis of malignant cells to the leptomeninges, cerebrospinal fluid (CSF) compartment, and subarachnoid space results in leptomeningeal disease (LMD)1. The prognosis of LMD is poor, with only 10% of solid tumor patients surviving beyond one year2,3,4,5. The advent of oncogene-directed therapies for patients with non-small-cell lung cancer (NSCLC) LMD has extended median survival from one month up to nearly one year1,6,7,8. Currently, diagnosis and response assessment in LMD relies upon CSF cytology, the clinical standard, as well as physical examination and magnetic resonance imaging (MRI) of the brain and spine9. CSF cytology is highly specific for LMD, but has a reported sensitivity of only 50–60%1,9,10,11.
Detection of tumor-derived DNA in CSF (CSF-tDNA) has emerged as a novel method for detecting and monitoring neoplastic processes of the central nervous system12,13,14,15,16,17,18, applying principles of peripheral blood-based circulating tumor DNA (ctDNA) detection to CSF. Many studies investigating the role of CSF-tDNA in LMD have been case reports16,19,20,21,22, and comprehensive studies with prospective cohorts remain rare23,24,25,26,27,28. The increasing number of potential genetic drivers in advanced-stage NSCLC prompted the development of next-generation sequencing (NGS)-based multiplex plasma ctDNA assays for the non-invasive detection of a wide range of genomic events. Recent cohort studies have demonstrated the feasibility of detecting CSF-tDNA in patients with EGFR-mutant and ALK-rearranged NSCLC27,28,29,30,31,32,33,34,35,36,37. Consistent observations across these reports are higher variant allele frequencies (VAFs) in CSF compared to plasma and better concordance between the original tumor and CSF-tDNA than between tumor and plasma ctDNA. Preliminary results suggest the sensitivity of CSF-tDNA is superior to that of CSF cytology23 and the utility of CSF-tDNA to predict treatment response remains unknown.
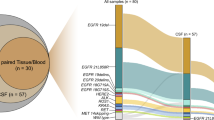
In the current study, we aimed to develop a CSF-tDNA assay using CAPP-Seq38,39,40,41,42, a targeted NGS-based method originally developed for the analysis of plasma ctDNA, for the diagnosis and monitoring of LMD in patients with lung cancer. We hypothesized that the detection of CSF-tDNA in patients with advanced lung cancer improves the sensitivity of LMD detection, enables analysis of treatment resistance mutations, and allows monitoring of LMD response to targeted therapy (Fig. 1).
Results
Detection of CSF-tDNA in lung cancer patients with suspected LMD
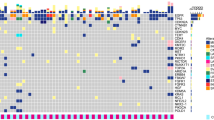
We profiled 81 CSF, blood, and tissue samples from 24 total patients with advanced lung adenocarcinomas who underwent lumbar puncture (LP) for evaluation of LMD (Supplementary Fig. 1). Median age of the patients in our cohort was 64 (IQR, 55.3–72.0). The majority of our cohort was female (N = 18) and did not have a history of smoking (N = 16). Most patients had concurrent brain metastases (N = 17) at the time of LMD evaluation. Our cohort was comprised of 20 patients with EGFR-mutant tumors, two patients with ALK-driven tumors, and two patients with non-EGFR and non-ALK mutated lung cancer (Fig. 2a). Eighteen patients had definitive LMD and six patients had possible LMD (see “Methods” for definitions). Reorganizing by EANO–ESMO criteria of confirmed, probable, and possible LMD, fourteen patients had confirmed LMD, four had probable LMD, and six had possible LMD. CSF-tDNA VAFs were significantly higher than plasma ctDNA VAFs (median CSF-tDNA, 32.7%; median plasma ctDNA, 1.8%; P < 0.0001; Fig. 2b). We further observed a significant positive correlation between CSF-tDNA VAF and plasma ctDNA VAF (Spearman’s ρ, 0.45; P = 0.03; Fig. 2c).
a Clinical characteristics and CSF-tDNA detection results. Each column represents a patient and each row a parameter (e.g., driver mutation). Similarly, the associated co-mutation plot depicts patient-level mutational profiles of CSF-tDNA in patients with lung cancer genotyped by our lung cancer-specific NGS panel. b Comparison of individual mutation VAFs in CSF-tDNA and in plasma ctDNA. P value calculated by the Mann–Whitney test. c Correlation of CSF-tDNA VAF with plasma ctDNA VAF. P value and ρ were calculated by Spearman correlation.
Comparison of CSF-tDNA to cytology and imaging for diagnosis of LMD
Classically, the detection of malignant cells in CSF by cytopathology is considered the gold standard for LMD diagnosis43. Of 12 patients with positive CSF by cytopathology or by a clinical EGFR PCR assay, CSF-tDNA was positive in 11 (92%). To account for this, we compared the sensitivity of cytology, MRI, and CSF-tDNA in detecting definitive LMD (see “Methods”). In our cohort, we found that cytology was 81.8% sensitive for the diagnosis of definitive LMD while MRI was only 80.0% sensitive. In contrast, CSF-tDNA was 91.7% sensitive for definitive LMD (Fig. 3a).
a Sensitivity of CSF-tDNA, cytology, and MRI in the diagnosis of LMD. b Comparison of CSF-tDNA VAF between cytology-positive and cytology-negative patients, stratified by which criteria were met in the definition of LMD. The cases who had negative cytology were diagnosed by MRI of the brain or spine with unequivocal evidence and progressive neurological symptoms consistent with LMD.
We next investigated the association between LMD and CSF-tDNA VAFs, including all samples with available CSF cytology, MRI, and history of neurological symptoms (N = 27). The mean CSF-tDNA VAF of cytology (+) samples (N = 18) was 15.3% while the mean VAF of cytology (−) samples was 17.4% (N = 9, P = 0.43) (Fig. 3b). Importantly, 4/9 samples which were cytology (−) had detectable CSF-tDNA (mean VAF, 39.1%). Of these four samples, two had both MRI findings of LMD and progressive neurologic symptoms at the time of LP and two had MRI findings alone.
Prognostic utility of CSF-tDNA for patients with suspected LMD
Next, we explored the prognostic value of CSF-tDNA detection in patients with definitive LMD (N = 17). We found that the presence of CSF-tDNA is strongly associated with poor OS (HR = 6.6; P = 0.03; Fig. 4a). Expanding across all samples with sufficient genoty** depth (N = 21), this association remained significant (HR = 7.5; P = 0.02; Fig. 4b). In this subset, thirteen patients had cytology confirmed LMD. Intriguingly, one patient was ctDNA negative in the same sample and this patient was subsequently lost to follow-up after 853 days without CNS progression. In clinical CSF cytology, centrifugation of the sample is commonly performed to collect cells for further processing and the supernatant is generally discarded43. CSF-tDNA isolation, as performed in this study, is compatible with cytology, as we centrifuged the CSF sample and extracted DNA from the supernatant. Thus, we tested the prognostic utility of considering both CSF cytology and CSF-tDNA. We found that patients who were CSF-tDNA and cytology positive had significantly worse overall survival (OS) than patients who were CSF-tDNA and cytology negative (HR = 8.4; P = 0.015; Supplementary Fig. 2a). Four patients had discordant CSF-tDNA and cytology (CSF-tDNA positive, cytology negative). The prognosis for these patients appears to be better than patients with both CSF-tDNA and cytology positive but worse than patients who are CSF-tDNA and cytology negative (Supplementary Fig. 2b).
a Kaplan–Meier curve comparing patients with definitive LMD and detectable (N = 14) and undetectable (N = 3) CSF-tDNA at first lumbar puncture for the endpoint of overall survival (P = 0.03, HR = 6.6 (95% CI, 2.1–20.7)). b The association remained prognostic when including patients with both definitive and possible LMD. P value and hazard ratio were calculated from the log-rank test.
Spatial heterogeneity of resistance mechanisms to targeted therapy
We next investigated the ability of time-matched CSF-tDNA and plasma ctDNA to detect resistance mechanisms to targeted therapy. In this analysis, we included EGFR-mutant lung adenocarcinoma patients who received EGFR tyrosine kinase inhibitor (TKI) therapy, had a pre-TKI sample available (CSF, N = 7; tumor biopsy, N = 1, pleural effusion, N = 1), and had available post-TKI therapy CSF and plasma samples, collected within two weeks of each other with no intervening treatment. To identify putative resistance mutations, we performed tumor-naïve variant calling39 on the post-TKI plasma sample, retaining mutations in genes known to be associated with resistance mutations (exonic SNVs, EGFR, PIK3CA, KRAS, CDKN2A, RB1, ALK, KIT, and MET; copy number variants, MET, ERBB2, and EGFR)40. Resistance mutations were defined as variants in these genes that were absent in the pre-TKI sample.
Seven patients (77%) in this analysis received osimertinib and two patients (22%) received erlotinib. Seven patients (77%) had CNS progression, one patient (11%) had non-CNS progression, and one patient (11%) did not have any progression after EGFR-TKI therapy. We detected putative resistance mutations in the peripheral blood of seven patients (77%), all of whom had CNS progression on EGFR-TKI therapy. Strikingly, resistance mutations were much less commonly observed in matched CSF though tumor DNA concentration was higher in CSF than plasma in the case (LUP112) where a shared resistance SNV was identified in both compartments. When we did detect emergent resistance mutations in the CSF (N = 2, MET amplification, and PIK3CA SNV) the same mutations were also observed in matched plasma (Fig. 5a). No resistance mutations, in either plasma or CSF, were detected in the two patients without CNS PD.
This analysis was limited to EGFR-mutant lung adenocarcinoma patients who received EGFR-TKI therapy, had a pre-TKI sample available, and had available post-TKI therapy CSF and plasma samples, collected within two weeks of each other with no intervening treatment (N = 9). a Clinical characteristics co-mutation plot of resistance mutations. PD progressive disease. b Patient (LUP112) with Stage IV adenocarcinoma with an emergent KRAS G12A mutation in plasma that was absent in the pre-treatment tumor. This mutation was absent in detected CSF-tDNA at the time of LMD diagnosis. c Patient (LUP132) with Stage IV adenocarcinoma with an emergent EGFR T790M mutation and ERBB2 amplification in plasma, both of which were absent in time-matched CSF.
We provide two illustrative cases to highlight these findings. In Fig. 5b, we present the case of a patient (LUP112) with Stage IV adenocarcinoma with known EGFR del19 and TP53 R248W mutations. This patient was treated with erlotinib and developed osseous metastases. A plasma sample at this time revealed both mutations, but also a KRAS G12A mutation that had been absent in the initial tumor. The patient was transitioned to a regimen of carboplatin, pemetrexed, and bevacizumab but went on to later develop worsening headaches and altered mental status. Though MRI did not reveal leptomeningeal enhancement, an LP was performed. Cytology was positive and CSF-tDNA was notable for high VAF detection of the original EGFR del19 and TP53 R248W mutations. A plasma sample collected the same revealed these two mutations plus KRAS G12A, suggesting the resistant clone did not enter the CNS. In Fig. 5c, we present the case of another patient (LUP132) with Stage IV adenocarcinoma with a known EGFR del19 mutation, detected in both tumor and plasma. This patient went on to develop progressive headaches and had an MRI concerning leptomeningeal enhancement. CSF-tDNA was detected in two consecutive LPs and EGFR del19 was detected in both. However, time-matched plasma samples revealed an emergent EGFR T790M mutation and emergent ERBB2 amplification, both of which were absent in CSF.
CSF-tDNA predicts response to osimertinib in a prospective cohort
A subset of our overall cohort was collected as part of a prospective study examining the efficacy of osimertinib for refractory LMD44. Patients (N = 12) underwent LPs, before and 3 weeks after initiating treatment with osimertinib. Follow-up LPs were accompanied by brain MRI and venipuncture for matched plasma ctDNA analysis. We found that patients with CNS progression had increased CSF-tDNA VAFs after three weeks of osimertinib (Fig. 6a). We next tested if CSF-tDNA VAFs varied with progression status. We found no difference between pre- and on-osimertinib CSF-tDNA VAFs among patients with CNS PD (P = 0.67) but a trend towards lower CSF-tDNA VAFs among patients without CNS PD (P = 0.08, Fig. 6b). CSF samples from the trial were also analyzed for osimertinib penetration rate (CSF osimertinib concentration normalized by plasma osimertinib concentration) and we therefore explored the association between drug penetration and on-treatment CSF-tDNA concentration. There was no significant correlation between osimertinib penetration rate and on-osimertinib CSF-tDNA concentration (Spearman’s ρ, 0.32; P = 0.30; Fig. 6c). Similarly, there was no significant correlation between osimertinib CSF penetration and the difference in on-osimertinib and pre-osimertinib VAF (Spearman’s ρ, 0.32; P = 0.46), as was the association between plasma osimertinib concentration and post-osimertinib plasma ctDNA VAF (Supplementary Fig. 3a, b). Finally, we investigated the utility of median CSF-tDNA VAFs, pre-osimertinib, and post-osimertinib, for predicting response to therapy. While there was no significant association between pre-osimertinib CSF-tDNA VAFs and CNS progression (Supplementary Fig. 4), on-osimertinib CSF-tDNA VAF was strongly associated with CNS progression on therapy (HR = 6.2, P = 0.009, Fig. 6d).
a Patients with both pre- and on-osimertinib CSF samples available (N = 7), percent change in CSF-tDNA VAF. b Comparison of CSF-tDNA VAF pre- and post-osimertinib in patients with CNS PD (P = 0.67) and patients without CNS PD (P = 0.08). P values were calculated by the Mann–Whitney test. c Correlation of CSF-tDNA VAF with percent CSF osimertinib penetration, colored by CNS progression status (red, CNS PD; black, no CNS PD). Percent CSF osimertinib penetration was calculated by normalizing CSF osimertinib concentration (nM) to plasma osimertinib concentration (nM). P value and ρ were calculated by Spearman correlation. d Kaplan–Meier curve comparing patients with post-osimertinib CSF-tDNA VAF above and below the median (4.8%) for the endpoint of freedom from CNS progression (P = 0.009, HR = 6.2 (95% CI, 1.2–31.8)). P value and hazard ratio were calculated from the log-rank test.
Discussion
In this study, we provide evidence for the utility of CSF-tDNA analysis in the management of NSCLC patients with LMD. We found that resistance mutations detected in plasma ctDNA are often absent from CSF-tDNA, suggesting an evolution of distinct resistance mechanisms based on the location of tumor deposits. Additionally, we found that CSF-tDNA detection at the time of LMD diagnosis and drop of CSF-tDNA concentration in response to targeted therapy with osimertinib appears to have prognostic value, potentially facilitating improved patient counseling and risk stratification.
We observed that the detection of CSF-tDNA may be more sensitive than current methods of LMD diagnosis. While CSF cytology is highly specific, it has a sensitivity of only 50–60% on initial LP1,9, and is therefore an imperfect gold standard. In our cohort, CSF-tDNA was 91.7% sensitive. Furthermore, 4/9 samples that were cytology-negative had detectable CSF-tDNA in suspected LMD patients.
A well-established barrier to the long-lasting effectiveness of molecular therapies is the inevitable emergence of sub-clonal tumor populations harboring resistance mutations. Prior studies in NSCLC have demonstrated spatiotemporal heterogeneity of canonical resistance mutations, potentially offering opportunities for therapeutic re-challenge schemes and selective targeting of specific body compartments45. EGFR-TKI re-challenge remains promising, with multiple studies evaluating re-administration of EGFR-targeted therapy after salvage cytotoxic chemotherapy46,47. In the selection of patients most likely to benefit from TKI re-challenge, plasma ctDNA, and CSF-tDNA could allow personalized surveillance of resistance mechanisms within and outside of the CNS27,31,32,33,48. Early changes in clonal composition, particularly those conferring therapeutic resistance, could aid clinical decision-making in identifying drugs most likely to be effective. Additionally, beyond the dynamic heterogeneity in tumor genotype over time, spatial heterogeneity may also help direct therapy48.
Secondary resistance EGFR mutations, such as T790M with first and second-generation EGFR-TKIs and C797S mutation with third-generation EGFR-TKI, are common causes of acquired resistance to EGFR-TKIs in EGFR-mutant lung cancer. However, these secondary EGFR mutations appear less frequently in CNS resistance samples from EGFR-mutant lung cancer patients27,32. One hypothesis regarding this observation is that limited penetration of targeted therapeutics into the CNS due to the blood-brain barrier results in different clonal selection pressures between the CNS and periphery. Another hypothesis is that resistance arises because of the different microenvironments in the periphery versus the CNS, such as due to differences in secreted factors. Consistent with this hypothesis, recent evidence suggests that the EGFR ligand amphiregulin induces resistance in an EML4-ALK rearranged lung cancer LMD mouse model, a finding that was also confirmed in human CSF samples49. Analogous mechanisms could potentially lead to EGFR TKI resistance.
In the subset of our patients who received osimertinib, a third-generation EGFR-TKI demonstrated to have improved CNS response50,51,52, previously described resistance mutations in LMD were observed, with MET copy number gain seen in the CSF-tDNA of 1/5 patients with CNS progression53. We observed that resistance mutations were more prevalent in time-matched peripheral blood than in CSF, consistent with prior reports27,32. While the mechanisms driving this observation were not assessed in our study, future investigations into this divergence are warranted. A compartment-specific framework may meaningfully inform the development of patient-specific therapeutic decision-making.
The assessment of clinical response in patients with LMD remains challenging due to the limitations of MRI imaging, the lack of standard evaluation criteria, and the relatively poor sensitivity of cytology28,54. Notably, none of these methods are quantitative. Several prior studies have demonstrated the potential promise of CSF-tDNA in advanced NSCLC patients with EGFR and EML4-ALK mutant tumors24,26,27,28,31. Notably, these studies did not include patients from prospective cohorts. In our prospective osimertinib cohort, we found that, while pre-treatment CSF-tDNA levels were not prognostic, mid-treatment CSF-tDNA after only 3 weeks of treatment were elevated in patients who went on to develop CNS progression. This is a key finding for future studies because it offers an opportunity to adapt therapy early on in patients destined to develop rapid clinical deterioration. For example, one could envision early therapeutic change or escalation (e.g., addition of radiotherapy) in patients who do not show a drop of CSF-tDNA concentration at the 3-week mark. Future prospective clinical trials will be required to test the utility of such an approach.
Prior manuscripts in other cancer settings have described the importance of ensemble mutational consideration when quantifying clinical response39,55,56,57. Prior explorations of molecular response to osimertinib relied in individual reporters such as CDK4 and EGFR alterations and even the lowest risk groups had a median time to progression of ~10–15 months. Furthermore, prior studies have near-uniformly relied on the digital readout of whether these individual alterations are detectable58. Such approaches may be susceptible to assay detection limit parameters compared to ensemble approaches for disease quantification. Our prospective cohort receiving osimertinib demonstrated robust risk stratification with the low-risk group not reaching 50% intracranial progression despite over two years of follow-up. Particularly for clinical applications involving therapeutic selection and timing, maximizing risk stratification is crucial, particularly for rapidly progressive pathologies like LMD.
Despite our findings, additional work is necessary to more thoroughly understand how spatiotemporal heterogeneity emerges in the context of LMD. Studies of tumor evolutionary history allow for the construction of patient-personalized phylogenetic trees visualizing the accumulation of genomic aberrations over time59. Future studies could recapitulate these findings in parallel analyses of the CNS and systemic compartments in patients with CNS tumor involvement to temporally resolve the differences observed in our study. Additionally, while we describe the landscape of peripheral blood- and CSF-associated genetic aberrations in LMD, additional functional studies are necessary to identify their significance in disease pathogenesis and to elucidate putative druggable targets.
There are some limitations to this study. The first is that the number of CSF samples at the time of resistance was very small because it is not standard practice to do LP at the time of resistance. The second is that the amount of CSF was relatively limited due to the potential adverse effects of large-volume CSF collection.
In conclusion, we provide evidence supporting the utility of CSF-tDNA detection to diagnose LMD in lung cancer patients. Assessment of CSF-tDNA has the potential to measure the molecular response of LMD to EGFR-TKI treatment at early treatment time points with implications for therapeutic decision-making. The use of dynamic measurement of plasma ctDNA and CSF-tDNA status and profiling of co-occurring gene alterations provides support for prospectively testing novel strategies for personalized management of this devastating clinical entity.
Methods
Study cohorts
The samples analyzed in this article were collected at two institutions between 2015 and 2020. Thirteen patients were recruited at Stanford Hospital and Clinics and underwent LP as part of routine clinical management of suspected LMD. Samples from twelve patients who were enrolled in a prospective study to determine the efficacy of osimertinib in the treatment of refractory LMD at the Institute of Biomedical Research and Innovation Hospital were also included44. In the prospective cohort, collected and analyzed samples included: a pre-osimertinib sample (CSF, N = 9; tumor, N = 1; pleural effusion, N = 2), a post-osimertinib CSF sample (N = 12), and a time-matched post-osimertinib plasma sample (N = 12). The post-osimertinib samples were collected either at the time of disease progression or at the last available follow-up. This prospective cohort is referred to as the “prospective osimertinib cohort” throughout the manuscript. In both cohorts, an MRI was obtained prior to the LP. Written informed consent was obtained from all patients and enrollment of each cohort was approved by the Institutional Review Board at each respective institution (retrospective cohort, Stanford University, United States of America; prospective cohort, Institute of Biomedical Research and Innovation Hospital, Kobe, Japan) and complied with the Declaration of Helsinki. All patient identifiers are anonymized prior to analysis and are not linkable to health data from the patients they represent. Detailed information regarding patients and samples is depicted in Supplementary Fig. 1. Clinical data is included in Supplementary Data 2.
Sample collection and processing
All plasma, tumor, and pleural effusion samples were analyzed by CAPP-Seq as previously reported41,42. Peripheral blood was collected in K2EDTA tubes and CSF was collected in standard, plastic LP collection tubes. Cell-free DNA was extracted from CSF samples by QIAamp Circulating Nucleic Acid Kit (QIAGEN, Hilden, Germany) using a protocol adapted from the methods defined by Pentsova et al. 15. Briefly, CSF was placed on ice after collection and centrifuged at 1800×g for 10 min. The supernatant was transferred to a second tube and then centrifuged again at 20,000×g for an additional ten minutes. Cell-free DNA was isolated and used in downstream applications in kee** with our previously described CAPP-Seq methodology41,42, described briefly below.
Library preparation and targeted NGS
DNA isolation, library preparation, and targeted sequencing were performed using iDES-enhanced CAPP-Seq previously described41,42. Briefly, plasma, CSF, and germline DNA (from peripheral blood mononuclear cells (PBMCs) in plasma-depleted whole blood) were used to build sequencing libraries and subjected to targeted exome capture using a previously published lung cancer CAPP-Seq selector60. Sequencing was performed on Illumina HiSeq 4000 instruments (Illumina, San Diego, CA) using 2× 150 paired-end reads with custom adapters for sample multiplexing and molecular barcoding. Sequencing reads were mapped to the human genome (build hg19) followed by the removal of PCR duplicates and technical artifacts as previously described41,42. CSF samples were sequenced to a median deduplicated depth of 150×, plasma samples were sequenced to a median deduplicated depth of 1879×, and germline samples were sequenced to a median deduplicated depth of 1099×. Sequencing data were processed using a custom bioinformatics pipeline and SNV, indel, and structural variant calling was performed as previously described41,42. In kee** with our previous work using iDES-enhanced CAPP-Seq41, cell-free DNA sequencing reads were de-duplicated using molecular barcodes, background-polished to reduce stereotyped base substitution errors, and filtered to limit the selector space.
Measurement of osimertinib levels
All CSF samples were collected after 6 ± 2 h from osimertinib administration and plasma samples were simultaneously collected. The CSF and plasma concentrations of osimertinib were measured using liquid chromatography-tandem mass spectrometry. The CSF penetration rate of osimertinib was estimated based on CSF/plasma concentrations.
Criteria for CSF-tDNA detection
Samples were analyzed for the presence of mutations using CAPP-Seq on plasma, CSF, or plasma-depleted whole blood without a priori knowledge of tumor mutations, as previously described39,41,42. SNPs were excluded via identification in germline or plasma and protein-coding mutations were retained. A joint set of mutations for each patient was then assessed as a group in each sample, and a Monte Carlo-based tumor DNA detection index was measured to determine significance (index cutoff point of ≤0.05), as previously established39,41,42. If the detection index was >0.05, plasma ctDNA or CSF-tDNA was classified as not detected at that time point, whereas if it was ≤0.05 it was classified as detected. The sample plasma ctDNA mutant allele fraction was calculated by averaging the mutant allele fractions for all mutations for that patient. All variant calls are listed in Supplementary Data 2.
Somatic copy number alteration detection
Somatic copy number alterations (SCNAs) were called using a previously described method40. In brief, SCNAs were detected using a z-score-based approach which involves a set of background samples to capture region-specific variabilities in depth across the targeted regions. For each gene, we called focal amplifications and deletions using the targeted regions as determined by the lung cancer CAPP-Seq panel.
Definition of definitive (1) and possible (2A and 2B) LMD
LMD diagnosis was considered definitive under the following conditions:
-
1. Positive CSF cytology or positive clinical EGFR CSF PCR in the initial LP or
-
2A. MRI of the brain or spine performed prior to the diagnostic LP with unequivocal evidence of LMD and
-
2B. Progressive neurological symptoms consistent with LMD, following exclusion of other possible causes.
MRIs were reviewed for evidence of LMD by a board-certified neuroradiologist (M.I.). This definition was adapted based on a prior study evaluating a flow cytometry-based method for LMD diagnosis61. We conducted secondary analyses using the EANO–ESMO criteria where confirmed LMD was defined by positive CSF cytology, probable LMD was defined by negative/equivocal cytology but positive MRI findings and symptoms, and possible LMD was defined as negative cytology and MRI but with concordant symptomology.
Statistics
Our primary aim was to test the hypothesis that detection of CSF-tDNA is associated with survival, thus our primary outcome was OS (event defined as death from any cause). In our study, all mortality events were attributable to LMD. In the prospective osimertinib cohort, we considered an additional survival endpoint, freedom from CNS progression. This was defined by radiographic and neurological progression. Neurological changes were evaluated by the following factors: disorientation (date and time, location, and name), headache, diplopia, blindness, paresthesia, gait disturbance, and grip strength. We also performed the finger-nose test, eye movement test, meningeal sign test, Barre test, and sense of touch test. Extra-CNS response was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. As CNS radiologic changes are difficult to assess by the RECIST, they were evaluated as improved, stable, and progressed based on findings of dura mater thickening, exuding contrast agent, ventricular distention, and/or, concomitant substantial brain metastases with confirmation by at least two doctors. Time-to-event analysis for survival endpoints was done using the log-rank test to estimate both P values and hazard ratios and expressed as Kaplan–Meier plots. Comparisons of the two groups were tested using the Mann–Whitney test. The strength of correlations between continuous variables was assessed using Spearman’s correlation coefficient. All statistical analyses were done using Prism 7 (GraphPad Software) or R v3.2.2 (http://www.r-project.org) through the RStudio environment.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Sequencing data supporting the findings are available on request from the lead corresponding author (Diehn) and are not deposited into a secure access-controlled repository as the institutional ethics committee does not allow for these data to be deposited into a secure access-controlled repository. Supporting variant-level data for all figures are available in the Supplement. Reasonable requests for data access from qualified researchers will be reviewed by the senior authors to determine whether they can be fulfilled in accordance with privacy restrictions via collaboration or data usage agreement.
Code availability
The computer code used for the analysis was described previously42 and is available at http://cappseq.stanford.edu.
References
Cheng, H. & Perez-Soler, R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol. 19, e43–e55 (2018).
Geukes Foppen, M. H. et al. Targeted treatment and immunotherapy in leptomeningeal metastases from melanoma. Ann. Oncol. 27, 1138–1142 (2016).
Kuiper, J. L. et al. Treatment and survival of patients with EGFR-mutated non-small cell lung cancer and leptomeningeal metastasis: a retrospective cohort analysis. Lung Cancer 89, 255–261 (2015).
Abouharb, S. et al. Leptomeningeal disease and breast cancer: the importance of tumor subtype. Breast Cancer Res. Treat. 146, 477–486 (2014).
Seute, T. et al. Leptomeningeal metastases from small cell lung carcinoma. Cancer 104, 1700–1705 (2005).
Nguyen, T. K. et al. Predictors of leptomeningeal disease following hypofractionated stereotactic radiotherapy for intact and resected brain metastases. Neuro Oncol. 22, 84–93 (2020).
Remon, J., Le Rhun, E. & Besse, B. Leptomeningeal carcinomatosis in non-small cell lung cancer patients: a continuing challenge in the personalized treatment era. Cancer Treat. Rev. 53, 128–137 (2017).
Li, Y.-S. et al. Leptomeningeal metastases in patients with NSCLC with EGFR mutations. J. Thorac. Oncol. 11, 1962–1969 (2016).
Nayar, G. et al. Leptomeningeal disease: current diagnostic and therapeutic strategies. Oncotarget 8, 73312–73328 (2017).
Pan, Z. et al. Leptomeningeal metastasis from solid tumors: clinical features and its diagnostic implication. Sci. Rep. 8, 10445 (2018).
Glantz, M. J. et al. Cerebrospinal fluid cytology in patients with cancer: minimizing false-negative results. Cancer 82, 733–739 (1998).
Miller, A. M. et al. Tracking tumour evolution in glioma through liquid biopsies of cerebrospinal fluid. Nature 565, 654–658 (2019).
Panditharatna, E. et al. Clinically relevant and minimally invasive tumor surveillance of pediatric diffuse midline gliomas using patient-derived liquid biopsy. Clin. Cancer Res. 24, 5850–5859 (2018).
Azad, T. D. et al. Liquid biopsy for pediatric diffuse midline glioma: a review of circulating tumor DNA and cerebrospinal fluid tumor DNA. Neurosurg. Focus 48, E9 (2020).
Pentsova, E. I. et al. Evaluating cancer of the central nervous system through next-generation sequencing of cerebrospinal fluid. J. Clin. Oncol. 34, 2404–2415 (2016).
De Mattos-Arruda, L. et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat. Commun. 6, 8839 (2015).
Wang, Y. et al. Detection of tumor-derived DNA in cerebrospinal fluid of patients with primary tumors of the brain and spinal cord. Proc. Natl. Acad. Sci. USA 112, 9704–9709 (2015).
Chiang, C.-L. et al. Cerebrospinal fluid as a medium of liquid biopsy in the management of patients with non-small-cell lung cancer having central nervous system metastasis. Front Biosci. (Landmark Ed.) 26, 1679–1688 (2021).
Song, Y. et al. Osimertinib quantitative and gene variation analyses in cerebrospinal fluid and plasma of a non-small cell lung cancer patient with leptomeningeal metastases. Curr. Cancer Drug Targets 19, 666–673 (2019).
Melms, J. C. et al. Implementation of cell-free tumor DNA sequencing from the cerebrospinal fluid to guide treatment in a patient with primary leptomeningeal melanoma: a case report. Mol. Clin. Oncol. 9, 58–61 (2018).
Li, Y. et al. Tumor DNA in cerebral spinal fluid reflects clinical course in a patient with melanoma leptomeningeal brain metastases. J. Neurooncol. 128, 93–100 (2016).
Pan, W. et al. Brain tumor mutations detected in cerebral spinal fluid. Clin. Chem. 61, 514–522 (2015).
White, M. D. et al. Detection of leptomeningeal disease using cell-free DNA from cerebrospinal fluid. JAMA Netw. Open 4, e2120040 (2021).
Chiang, C.-L. et al. Utility of cerebrospinal fluid cell-free DNA in patients with EGFR-mutant non-small-cell lung cancer with leptomeningeal metastasis. Target Oncol. 16, 207–214 (2021).
Angus, L. et al. Detection of aneuploidy in cerebrospinal fluid from patients with breast cancer can improve diagnosis of leptomeningeal metastases. Clin. Cancer Res. 27, 2798–2806 (2021).
Ma, C. et al. Detection of circulating tumor DNA from non-small cell lung cancer brain metastasis in cerebrospinal fluid samples. Thorac. Cancer 11, 588–593 (2020).
Li, Y. S. et al. Unique genetic profiles from cerebrospinal fluid cell-free DNA in leptomeningeal metastases of EGFR-mutant non-small-cell lung cancer: a new medium of liquid biopsy. Ann. Oncol. 29, 945–952 (2018).
Xu, Y. et al. Prospective study revealed prognostic significance of responses in leptomeningeal metastasis and clinical value of cerebrospinal fluid-based liquid biopsy. Lung Cancer 125, 142–149 (2018).
Li, N. et al. Prognostic significance of molecular characteristics of cerebrospinal fluid for non-small cell lung cancer patients with leptomeningeal metastasis. Thorac. Cancer 10, 1673–1682 (2019).
Huang, R. et al. Digital PCR-based detection of EGFR mutations in paired plasma and CSF samples of lung adenocarcinoma patients with central nervous system metastases. Target Oncol. 14, 343–350 (2019).
Zheng, M.-M. et al. Clinical utility of cerebrospinal fluid cell-free DNA as liquid biopsy for leptomeningeal metastases in ALK-rearranged NSCLC. J. Thorac. Oncol. 14, 924–932 (2019).
Wijetunga, N. A. et al. Dynamic mutational landscape of cerebrospinal fluid circulating tumor DNA and predictors of survival after proton craniospinal irradiation for leptomeningeal metastases. Clin. Cancer Res. 29, 775–783 (2023).
Yang, H. et al. Cerebrospinal fluid-derived circulating tumor DNA is more comprehensive than plasma in NSCLC patients with leptomeningeal metastases regardless of extracranial evolution. Heliyon 8, e12374 (2022).
Wang, Y. et al. Unique genomic alterations of cerebrospinal fluid cell-free DNA are critical for targeted therapy of non-small cell lung cancer with leptomeningeal metastasis. Front Oncol. 11, 701171 (2021).
Nie, N. et al. Genoty** of cerebrospinal fluid in lung cancer patients with leptomeningeal metastasis. Thorac. Cancer 13, 2574–2583 (2022).
Chiang, C.-L. et al. Efficacy of different platforms in detecting EGFR mutations using cerebrospinal fluid cell-free DNA from non-small-cell lung cancer patients with leptomeningeal metastases. Thorac. Cancer 14, 1251–1259 (2023).
Wu, X. et al. Cerebrospinal fluid cell-free DNA-based detection of High Level of genomic instability is associated with poor prognosis in NSCLC patients with leptomeningeal metastases. Front Oncol. 12, 664420 (2022).
Azad, T. D. et al. Circulating tumor DNA analysis for detection of minimal residual disease after chemoradiotherapy for localized esophageal cancer. Gastroenterology 158, 494–505.e6 (2020).
Chaudhuri, A. A. et al. Early detection of molecular residual disease in localized lung cancer by circulating tumor DNA profiling. Cancer Discov. 7, 1394–1403 (2017).
Chabon, J. J. et al. Circulating tumour DNA profiling reveals heterogeneity of EGFR inhibitor resistance mechanisms in lung cancer patients. Nat. Commun. 7, 11815 (2016).
Newman, A. M. et al. Integrated digital error suppression for improved detection of circulating tumor DNA. Nat. Biotechnol. 34, 547–555 (2016).
Newman, A. M. et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat. Med. 20, 548–554 (2014).
Torzewski, M. et al. (eds) Integrated Cytology of Cerebrospinal Fluid (Springer, 2008).
Nanjo, S. et al. Standard-dose osimertinib for refractory leptomeningeal metastases in T790M-positive EGFR-mutant non-small cell lung cancer. Br. J. Cancer 118, 32–37 (2018).
Hata, A. et al. Spatiotemporal T790M heterogeneity in individual patients with EGFR-mutant non-small-cell lung cancer after acquired resistance to EGFR-TKI. J. Thorac. Oncol. 10, 1553–1559 (2015).
Tomizawa, Y. et al. Effect of gefitinib re-challenge to initial gefitinib responder with non-small cell lung cancer followed by chemotherapy. Lung Cancer 68, 269–272 (2010).
Costa, D. B. et al. Effects of erlotinib in EGFR mutated non-small cell lung cancers with resistance to gefitinib. Clin. Cancer Res. 14, 7060–7067 (2008).
Gao, T., Chen, F. & Li, M. Sequencing of cerebrospinal fluid in non-small-cell lung cancer patients with leptomeningeal metastasis: a systematic review. Cancer Med. 12, 2248–2261 (2023).
Arai, S. et al. Osimertinib overcomes alectinib resistance caused by amphiregulin in a leptomeningeal carcinomatosis model of ALK-rearranged lung cancer. J. Thorac. Oncol. 15, 752–765 (2020).
Wu, Y.-L. et al. CNS efficacy of osimertinib in patients with T790M-positive advanced non-small-cell lung cancer: data from a randomized phase III trial (AURA3). J. Clin. Oncol. 36, 2702–2709 (2018).
Ramalingam, S. S. et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N. Engl. J. Med 382, 41–50 (2020).
Reungwetwattana, T. et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J. Clin. Oncol. 36, JCO2018783118 (2018).
Nanjo, S. et al. MET copy number gain is associated with gefitinib resistance in leptomeningeal carcinomatosis of EGFR-mutant lung cancer. Mol. Cancer Ther. 16, 506–515 (2017).
Le Rhun, E. et al. The RANO Leptomeningeal Metastasis Group proposal to assess response to treatment: lack of feasibility and clinical utility and a revised proposal. Neuro Oncol. 21, 648–658 (2019).
Shah, A. T. et al. A comprehensive circulating tumor DNA assay for detection of translocation and copy-number changes in pediatric sarcomas. Mol. Cancer Ther. 20, 2016–2025 (2021).
Kurtz, D. M. et al. Dynamic risk profiling using serial tumor biomarkers for personalized outcome prediction. Cell 178, 699–713.e19 (2019).
Kurtz, D. M. et al. Circulating tumor DNA measurements as early outcome predictors in diffuse large B-cell lymphoma. J. Clin. Oncol. 36, 2845–2853 (2018).
Zheng, M.-M. et al. Genoty** of cerebrospinal fluid associated with osimertinib response and resistance for leptomeningeal metastases in EGFR-mutated NSCLC. J. Thorac. Oncol. 16, 250–258 (2021).
Abbosh, C. et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature 545, 446–451 (2017).
Chabon, J. J. et al. Integrating genomic features for non-invasive early lung cancer detection. Nature 580, 245–251 (2020).
Milojkovic Kerklaan, B. et al. EpCAM-based flow cytometry in cerebrospinal fluid greatly improves diagnostic accuracy of leptomeningeal metastases from epithelial tumors. Neuro Oncol. 18, 855–862 (2016).
Acknowledgements
This work was supported by grants from the National Cancer Institute (M.D. and A.A.A., R01CA188298 and R01CA254179), the US National Institutes of Health Director’s New Innovator Award Program (M.D.; 1-DP2-CA186569), the Virginia and D.K. Ludwig Fund for Cancer Research (M.D. and A.A.A.), the CRK Faculty Scholar Fund (M.D.), the Doris Duke Charitable Foundation (T.D.A.), the Japan Society for the Promotion of Science KAKENHI (S.N. 21K15546 and 23K06648), and Japanese respiratory society fellowship (S.N.). Visualizations created with BioRender.com.
Author information
Authors and Affiliations
Contributions
Study concept and design: All authors. Acquisition of data: T.D.A. and S.N. Analysis and interpretation of data: T.D.A., S.N., M.C.J., and M.D. Drafting of the manuscript: all authors. Critical revision of the manuscript: all authors. Technical and material support: S.N., A.A.A., M.D., and M.H.G. Study supervision: A.A.A. and M.D. Accountability for all aspects of work: all authors.
Corresponding authors
Ethics declarations
Competing interests
A.M.N., A.A.A., and M.D. are co-inventors on patent applications related to cancer biomarkers. A.M.N. reports consultancy with CiberMed. A.A.A. and M.D. are consultants for Genentech and Roche. J.J.C. served as a consultant with Lexent Bio. A.A.A. has served as a consultant for Chugai, Gilead, and Celgene. A.A.A. reports ownership interest in CiberMed and FortySeven. M.D. has served as a consultant for AstraZeneca, Novartis, Bristol Myers Squibb, Gritstone Oncology, and Boehringer Ingelheim. M.D. and A.A.C. have received research funding from Varian Medical Systems. M.D. has received research funding from Illumina. S.N. has received research funding from AstraZeneca. M.D. and A.A.A. report ownership interests in CiberMed and Foresight Diagnostics.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Azad, T.D., Nanjo, S., **, M.C. et al. Quantification of cerebrospinal fluid tumor DNA in lung cancer patients with suspected leptomeningeal carcinomatosis. npj Precis. Onc. 8, 121 (2024). https://doi.org/10.1038/s41698-024-00582-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41698-024-00582-1
- Springer Nature Limited