Abstract
Due to the BCS theory, hydrogen, the lightest element, would be the prospect of room-temperature superconductor after metallization, but because of the difficulty of the hydrogen metallization, the theory about hydrogen pre-compression was proposed that the hydrogen-rich compounds could be a great option for the high Tc superconductors. The superior properties of TmH6, YbH6 and LuH6 indicated the magnificent potential of heavy rare earth elements for low-pressure stability. Here, we designed XTmH12 (X = Y, Yb, Lu, and La) to obtain higher Tc while maintaining low pressure stability. Most prominently, YbTmH12 can stabilize at a pressure of 60 GPa. Compared with binary TmH6 hydride, its Tc was increased to 48 K. The results provide an effective method for the rational design of moderate pressure stabilized hydride superconductors.
Similar content being viewed by others
Introduction
Since Kamerlingh Onnes discovered that mercury (Hg) suddenly starts carrying a current without resistance at an extremely low temperature in 19111,2, the achievement of room temperature superconductor is a dream for the superconductivity research. The theory that hydrogen can be metallized at high pressure was developed in 1935 and was proposed by Winger and Huntington3. According to the theory of superconductivity proposed by Bardeen, Cooper and Schrieffer in 1957, the transition temperature of superconductivity is proportional to the Debye temperature4. Due to this theory, hydrogen, the lightest element, would be the prospect of room-temperature superconductor after metallization5, but because of the difficulty of the hydrogen metallization6,7, the theory about hydrogen pre-compression was proposed by Ashcroft that the hydrogen-rich compounds could be a great option for the high Tc superconductors8,9. The theory of chemical pre-compression refers to the addition of other elements to the synthesized hydrogen-rich compounds at a lower pressure than synthesizing pure hydrogen10. Based on this conclusion, many great hydrogen-rich compounds have been designed and predicted to be potential superconductors with high Tc11,12,13. The first successful predictions were H3S and LaH10 with high Tc exceeding 200 K14,15,16, and these predictions were successfully confirmed by experiment soon17,18,19,20.
Over these years, with the efforts of our researchers, almost all binary hydrides were explored, people commence the study of ternary hydride formed by adding a new element into binary hydrides. In 2019, Li2MgH16 with the highest Tc to date (473 K at 250 GPa), designed by filling the anti-bonding orbital of the H2 molecular unit of MgH16 with the element Li21. H–C–S compounds and Lu–N–H compounds have been widely studied for some time due to the claimed observation of room temperature superconductivity. However, there are still some controversial issues about the stoichiometry and the crystal structure22,23,24,25. Recently, a new kind of fluorite-type clathrate ternary hydrides AXH8 (A = Ca, Sr, Y, La, X = B, Be, Al) in the main chain of hydrogen alloys has been predicted26. The most prominent, LaBeH8, is dynamically stable down to 20 GPa and has a high Tc up to 185 K. The exciting thing is that the cubic clathrate superhydrides LaxY1-xH6,10 have been experimentally synthesized by laser heating of yttrium-lanthanum alloys, which exhibited a maximum critical temperature Tc of 253 K without increasing pressure27. According this experiment, it is practicable to incorporate a metal element in the clathrate hydride to keep the compounds steadily.
It is a widespread attention about the prominent superconductivity of the clathrate hydrides. Clathrate hexahydrides Im-3 m-XH6 (X = Mg, Ca, Sc, Y, La, Tm, Yb, Lu) are widespread in alkaline earth and rare earth metal superhydrides16,28,29,30,31,32. In this structure, there is a body-centered cube (bcc) with center occupied by a metal atom, and there is a H24 cage of hydrogen atoms in the void of the bcc lattice. CaH6 and YH6 have been experimentally synthesized with high Tcs of 215 K at 172 GPa33,34 and 227 K at 166 GPa, respectively35. Theoretically predicted Tcs of MgH6, ScH6 and LaH6 are 260 K at 300 GPa, 147 K at 285 GPa and174 K at 100 GPa, respectively. YbH6 and LuH6 in full 4f.-orbital shells are predicted to exhibit high Tc superconductivity at relatively low pressures (145 K, 70 GPa vs. 273 K, 100 GPa, respectively)32. With unfilled 4f. orbitals, TmH6 is stable at 50 GPa, but has a relatively low Tc at 25 K. There was a report that the structures of superhydrides at low pressure could keep stable by f electrons, such as lanthanide clathrate hydrides CeH936, PrH937 and NdH938. Although the filling of the metal atoms’ f orbital could make the structure more stable at low pressure, according to current research results, the Tcs of hydrides with unfilled 4f. orbitals are mostly very low.
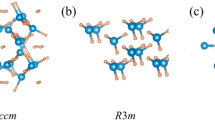
The properties of TmH6, YbH6 and LuH6 indicated the magnificent potential of such structures for low-pressure stability. In alkaline earth and rare earth metals hydrides Im-3 m-XH6 are common, such as CaH628, MgH629, YH615,16,30, ScH631, (Tm/Yb/Lu)H632. The structure can also be extended into the ternary structure Pm-3 m-ABH12, such as (Y,Ca)H6
To make the prediction more reliable, evaluation of the impact of the electron correlation effects is desired. Therefore, we calculated the band structure using U = 5 eV to figure out how Hubbard-U may modify the band structure (see Fig. S1 in Supplementary Material). After considering U = 5 eV, one flat band is lifted up into the unoccupied regime. This means that the occupation of 4f states is changed, that could have substantial impact on the electron–phonon coupling physics. Future studies will focus on the impact of U to pairing strength.
Discussion
Then, we have compared the electronic density of states at the Fermi level (NEf) in YTmH12, YbTmH12, LuTmH12 and LaTmH12 and binary hexahydrides, including YH6, TmH6, YbH6, LuH6, LaH6, as shown in Table 1. Benefit by 4f electrons from heavy rare earth elements Tm, large electronic density of states at the Fermi level in the XTmH12 is observed, much higher than that of the binary hexahydrides. The large H-derived electronic density of states at the Fermi level is beneficial for strong electron–phonon coupling (EPC) parameter λ. Generally speaking, NEf indicates all of the candidate electrons to form Cooper pairs. It is clear that the large NEf plays a positive role in enhancing the EPC λ. However, from the aspect of partial DOS, the contribution from H to the electronic density of states at the Fermi level in XTmH12 is not higher than the cases in binary hexahydrides. This suggests that the 4f electrons will play no role in superconductivity. The contrasting EPC λ in these clathrate hexahydrides is mainly attributed to the disparate intensity of H electrons interacting with optic phonons, rather than the contributions from global electronic structures. Papaconstantopoulos et al. apply the Gaspari-Gyorffy theory to determine that, in CaH6, the acoustic modes associated with Ca contribute only 7% to the total value of λ, in contrast to the optic modes associated with hydrogen which contribute 93% for the H49. And in LaH10, La has only a 2% contribution50.
The calculated Tcs by the Allen-Dynes modified McMillan equation51 are shown in Table 1. LaTmH12 has a Tc of 19–24 K at 170 GPa. This is not only much higher than the minimum stabilization pressure of 50 GPa for TmH6, but also higher than the pressure of 100 GPa for LaH6. LaTmH12 requires higher pressures to remain stable, probably due to the excessive gap between the properties of La and Tm. This type of ternary clathrate structure requires the two metal elements to be close in radius and other properties to ensure H cage stability. Thus, YTmH12 is able to stabilize at 80 GPa and exhibited Tc of 40–46 K. Both the minimum stabilization pressure and Tc are intermediate between the binary hydrides YH6 and TmH6. Yb and Lu, which are also heavy rare earth elements adjacent to Tm, have f electrons that can similarly enhance chemical pre-compression, so the stabilization pressure of their doped structures can be reduced even further, and YbTmH12 and LuTmH12 can be stabilized at 60 and 70 GPa, respectively, and exhibited Tc of 42–48 K. Their minimum stabilizing pressures and Tc also show a pattern intermediate to that of the binary hydrides.
Charge transfer has an important effect on the structure and properties of hydrides. Table 2 shows charges transferred for all thulium substituted clathrate hexahydrides. The e represents the total remaining electrons. Negative δ mean loss of electrons, positive δ mean gain of electrons. It can be seen that La is a very good electron donor and is able to provide sufficient electrons to the surrounding H. In LaTmH12, each La atom can provide 2.25 electrons, ultimately making 0.21 electrons available for each H on average. However, the provision of sufficient electrons does not necessarily mean that superconductivity is favored, and may even create factors that are detrimental to superconductivity. In terms of charge transfer, the ability of Tm to provide electrons is stronger than that of Y and Yb, but unfortunately, the presence of f electrons severely constrains higher Tc in thulium substituted clathrate hexahydrides.
To determine the origin of the superconductivity in these superconductors, we calculated their phonon spectrum, projected phonon density of state (PHDOS), integral EPC parameter λ and Eliashberg spectral function α2F(ω). The superconductivity of superconductors comes mainly from strong electron–phonon coupling (EPC). So, we can look for the frequency range in which the EPC parameter λ grows rapidly, and vibration modes in this frequency range are the key to the superconductivity of this structure. As can be easily seen in Fig. 4, λ grows rapidly in two regions: the low-frequency region and the mid-frequency region. For example, in LuTmH12, λ grows rapidly to 0.25 in the frequency range of 0–150 cm−1 and then grows slowly until the frequency range of 400–1000 cm−1, where λ grows rapidly to 1.1, and then grows hardly at all (see Fig. 4a). In YbTmH12, λ also grows rapidly in the frequency range of 500–1000 cm−1 (see Fig. 4b). By comparing the PHDOS of different elements, we can find the reason for the rapid growth of λ. The rapid growth of λ is mainly due to the vibrations of metal atoms in the low-frequency region (red and black peaks in PHDOS), while in the mid-frequency region it is due to the vibrations of hydrogen atoms (blue peaks in PHDOS). This corresponds to the two main sources of superconductivity in such clathrate hydrides: hydrogen on the hydrogen cage and the central metallic atom. In addition to this, it can be seen in Fig. 4c that the λ of YTmH12 grows rapidly when the frequency is 300 cm−1. On the phonon dispersion, there are soft phonon patterns near the R direction in this frequency range. This suggests that the softening of the optical branch of the phonon spectrum is also an important source of electron–phonon coupling. In LuTmH12, λ grows rapidly to 0.25 in the frequency range of 0–150 cm−1 consistent with YTmH12 (see Fig. 4d). However, in higher frequency range, λ grows much slower than that in YTmH12, which leads to the low Tc in LaTmH12.
In this work, we introduce other elements to improve the superconductivity of TmH6, allowing the newly formed thulium substituted clathrate hexahydrides XTmH12 (X = Y, Yb, Lu and La) to have the higher Tc while maintaining low-pressure stability. Most prominently, YbTmH12 can be stabilized at a pressure of 60 GPa. Its Tc is elevated compared to the binary TmH6, reaching 48 K. The results provide an effective method for the successful design of hydride superconductors at moderate pressures.
Computational methods
The candidate phases of XTmH12 (X = Y, Yb, Lu, and La) are predicted using the ab initio Random Structure search (AIRSS) technique52,53. The selected cut-off energy of the projected augmented wave (PAW)54 is 400 eV. The sampling density of the Brillouin district is 2π × 0.07 Å−1.The ultra-soft potentials is dynamically generated by the method of pseudo-potentials. The valence electrons in the electronic states of Y, Tm, Yb, Lu and La atoms are 4s24p65s24d1, 4f135s25p66s2, 4f145s25p66s2, 4f145p65d16s2, 5s25p65d16s2, respectively.
Structural relaxation, calculations of enthalpies, band structures, density of states and charge transfer of XTmH12 (X = Y, Yb, Lu and La) at different pressures were calculated by the Cambridge Serial Total Energy Package (CASTEP)55. We use the generalized gradient approximation (GGA)56 with the Perdew-Burke-Ernzerh of (PBE) parametrization57 as the exchange–correlation function. For the plane wave, we chose a cut-off energy of 800 eV. The sampling density of the Brillouin region is 2π × 0.03 Å−1. The pseudo-potential is dynamically generated by the ultra-soft potential.
Phonon dispersion, electron–phonon coupling and Eliashberg spectral function α2F(ω) of XTmH12 (X = Y, Yb, Lu and La) were calculated by the Quantum-ESPRESSO (Open-Source Package for Research in Electronic Structure, Simulation, and Optimization)58. With an ultra-soft potential and a cut off energy of 90 Ry, all XTmH12 (X = Y, Yb, Lu, La) in the first Brillouin region have a k-point grid of 12 × 12 × 12 and a q-point grid of 4 × 4 × 4, respectively. The superconducting transition temperatures of XTmH12 (X = Y, Yb, Lu and La) are estimated through the Allen−Dynes-modified McMillan equation (A-D-M) with correction factors51,59:
λ and ωlog are given by:
\(\lambda =2{\int }_{0}^{\infty }\frac{{\alpha }^{2}F(\omega )}{\omega }d\omega\) and \({\omega }_{log}=exp\left(\frac{2}{\lambda }{\int }_{0}^{\infty }\frac{d\omega }{\omega }{\alpha }^{2}F(\omega )\mathit{ln}\omega \right)\)
f1 and f2 are given by:
\({f}_{1}=\sqrt[3]{\left[1+{\left(\frac{\lambda }{2.46(1+3.8{\mu }^{*})}\right)}^\frac{3}{2}\right]}\) and \({f}_{2}=1+\frac{\left(\frac{{\omega }_{2}}{{\omega }_{log}}-1\right){\lambda }^{2}}{{\lambda }^{2}+\left[1.82(1+6.3{\mu }^{*})\frac{{\overline{\omega }}_{2}}{{\omega }_{log}}\right]}\)
average frequencies \({\overline{\omega }}_{2}\) is given by:
The typical value of Coulomb pseudo-potential μ* was set as 0.1–0.13.