Abstract
In this study, we present a method for synthesizing Ni-rich LiNi0.93Co0.04Al0.03O2 (NCA) with a high-energy cathode material by the solid-phase method. The sintering temperature plays a very important role in the electrochemical performance of the LiNi0.93Co0.04Al0.03O2 since it affects the crystallinity and structural stability. Therefore, various sintering temperatures (660 °C/690 °C/720 °C/750 °C/780 °C/810 °C) are studied to get optimum electrochemical performances. The electrochemical performance of LiNi0.93Co0.04Al0.03O2 sintered at 720 °C shows the highest discharge capacity of 217.48 mAh g−1 with excellent Coulombic efficiency of 87.84% at 0.1 C. Moreover, the LiNi0.93Co0.04Al0.03O2 sintered at 720 °C exhibits excellent rate-capability (181.1 mAh g−1 at 2.0 C) as well as superior cycle stability (95.4% after 80 cycles at 0.5 C). This is because optimized sintering temperature leads to good structural stability with low cation disorder and residual lithium content.
Similar content being viewed by others
Introduction
Recently, a rechargeable lithium-ion batteries (LIBs) have been widely used in various portable electronic devices and small mobile information technology (IT) devices. The high-capacity, energy density and power density are required for next-generation energy storage devices1,2,3,4. This is because, the application of LIBs has extended from small mobile IT devices to electric vehicles (EVs), hybrid electric vehicles (HEVs), and plug-in HEVs5.
LiCoO2 (LCO), the most common cathode material for LIBs, has been widely used because of its high coefficient of lithium ion diffusion, easy fabrication processing and high voltage6,7. However, LCO has major limitations such as low capacity, high cost, toxicity, poor rate capability and environmental pollution8,9. Especially, high energy and power density are essential towards in application of large-scaled devices such as EVs and HEVs. Therefore, many researchers have been focused on searching new cathode materials, which can have high power and energy density10,11,12,13. Among various candidates, LiNiO2 (LNO) cathode materials are being studied as an alternative to solve these problems such as high price and low capacitance of LCO. However, LNO, having low structural stability, is deformed from ‘layered structure’ to ‘spinel structure’ as the cycle progresses, finally resulting in slow lithium ion kinetics14,15.
To address these concerns, the LiNi1−y−zCoyAlzO2 (NCA) has been developed as a breakthrough16. It has also been reported that increasing the Al content provides higher capacity with stable performances7,17,18,19,20. The structural stability and the electrochemical performance of LNO can be improved by Co and Al substitutions for Ni site. Owing to smaller size of Co3+ (0.685 Å) and Al3+ (0.530 Å) than Ni3+ (0.740 Å), the substitution of Co and Al ions can lead to shrinkage of the a-axis, resulting in better stabilization of the layered structures which presents both high specific energy and power density.
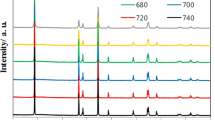
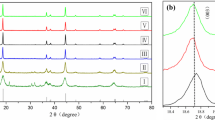
Therefore, in this study, we synthesized NCA cathode material with various sintering temperatures (660–810 °C) to optimize the electrochemical performances. This is because crystallinity and structural stability, affected by sintering temperature, play an important role in the electrochemical performances of NCA12,3g. It indicates that desired composition of NCA has been successfully synthesized.
Figure 4a shows the initial charge–discharge curves of NCA with different sintering temperatures at current density of 0.5 A/g in the voltage range of 3.0–4.3 V. The voltage plateau around 4.2 V is due to the high Ni ion concentration in the NCA26. We can confirm that the capacity of all samples except 660 ℃ and 810 °C is not significantly different. Therefore, the sintering temperature does not significantly affect the initial charge–discharge capacities in the range from 690 to 780 °C. The sample sintered at 720 °C shows the highest discharge capacity of 217.48 mAh g−1 with excellent Coulombic efficiency of 87.84%. This is due to the low cation mixing and well-crystallized layered structure, as mentioned earlier (Fig. 1). Figure 4b presents the rate performance of NCA with different C-rates (0.1, 0.5, 1.0 and 2.0 C) in voltage range of 3.0–4.3 V. All the rate performances were measured at a fixed charge current density of 0.5 C while the discharge current density was measured at different current densities. The cell was cycled at each rate and then back to 0.5 C. The rate capabilities of all samples are decreased with increasing C-rate. However, it can be seen that the difference between the capacity values of samples increases, as the C-rate increases. Compared to the other cases, the NCA sintered at 720 °C maintains the highest capacities regardless of C-rates. More importantly, the NCA sintered at 720 °C still has the highest capacity of 195.3 mA h g−1 when the C-rate is decreased back to 0.5 C, indicating superior reversibility. This is because well-defined layered structure via optimized sintering temperature allows electrochemically active {010} planes of lithium ions and electron conduction despite the fast charge/discharge rate, resulting from high cation ordering and highly crystallized layered structure.
Figure 5 shows the cycle performances of NCA samples at 0.5 C in the voltage range 3.0–4.3 V. No obvious capacity fading is observed for all samples before 45 cycles. However, there is a difference in the rate of decrease for each sample after 45 cycles. Among the samples at different sintering temperatures, the NCA sintered at 720 °C possesses the highest capacity with capacity retention of 95.4% after 80 cycles. It can be explained by not only the rapid/durable electrochemical kinetics but also interfacial stability between electrode/electrolyte. On the other hand, the NCA sintered at 660 °C and 810 °C shows inferior cyclabilities compared to others. The samples sintered at 660 °C and 810 °C show a sharp decrease in capacity upon cycling. This is due to inferior crystallization (660 °C), disordered layered structure (660 °C and 810 °C), and longer Li ion-transfer channels (810 °C). Such drawbacks cause sudden drop in capacity during cycling since they could destroy the structural integrity of NCA, resulting in reduced reactivity of active material. It was reported that the hydrogen fluoride (HF), originated from reaction of water and LiPF6, is one of the most important factor for performance degradation. This is because HF elutes the transition metal ions in NCA, leading to deformation of layered structure. The optimum sintering temperature can suppress the negative effects on the NCA via boosting structural stability.
To better understand the electrochemical performances, EIS tests of NCA sintered at 660 °C and 720 °C are performed. Nyquist plots for the both samples after 1st and 80th cycles are shown in Fig. 6a and b. It is well known that Nyquist plots is composed of three components: electrolyte resistance (Rs) in the high frequency, the charge transfer resistance (Rct) in medium frequency and the Warburg impedance in the low frequency26,27,28. Among three components, the Rct can be regarded as a key parameter for the cathode impedance, affecting the electrochemical behavior. The Rs values of both samples are almost same since they use the same electrolyte. However, there is significantly difference in Rct values between 1 and 80 cycles for both samples. Among them, the increase in Rct value of NCA sintered at 660 °C (174.4 Ω to 295.9 Ω) is much larger compared to that of NCA sintered at 720 °C, as shown in Table 2. It can be inferred that highly crystallized layered NCA offers enlarged exposed active planes for Li ions. The Rct value of NCA sintered at 720 °C shows about two-thirds than NCA sintered at 660 °C, resulting from enhanced charge-carrier transport at the NCA surface. Therefore, it can be concluded that optimized sintering temperature is beneficial to suppress the capacity fading during cycling based on structural stability of NCA.
Figure 7 shows the HCl titration curves of NCA with different sintered samples. The residual lithium compounds (Li2CO3 and LiOH) is derived from (i) moisture absorption and (ii) spontaneous reduction of Ni3+ into Ni2+, accompanied by oxygen release. The amount of HCl used in the HCl titration up to pH 4 of NCA sintered at 660 °C is higher compared to that of 720 °C, as shown in Table 3. The amount of Li2CO3 and LiOH can be calculated via following equations29,30:
The Li2CO3 is produced on the surface of LiOH by reaction with CO2, and then moisture remains, as follows:
Also, the CO2 and POF3 gases are generated by reaction with Li2CO3 and LiPF6. It can be represented by the following equation:
A portion of lithium is originated from the interior of NCA with the enhanced cation disordering. Therefore, the amount of residual lithium on the surface for sample sintered at 720 °C is small due to optimum sintering temperature31. It indicates that appropriate sintering temperature can effectively suppress the gassing and performance decay due to smooth surface chemistry without unwanted materials.
Conclusion
In this study, we successfully synthesized the Ni-rich LiNi0.93Co0.04Al0.03O2 cathode materials with low Li+/Ni2+ disorder and high crystallinity under various sintering temperature conditions (660–810 °C). The effect of sintering temperature on the structural properties and electrochemical performances of NCA were investigated. The results indicate that the electrochemical performances of NCA are effected by sintering temperature. Among various sintering temperatures, NCA sintered at 720 °C shows the highest electrochemical performances based on excellent structural stability. It can be elucidated that NCA sintered at 720 °C has a good layered structure with high cation ordering thereby allowing fast and smooth Li ion and electron diffusion. Therefore, we can conclude that NCA sintered at 720 °C could be used as a promising cathode material for next-generation high-energy LIBs.
References
Wu, F. & Yushin, G. Conversion cathodes for rechargeable lithium and lithium-ion batteries. Energy Environ. Sci. 10, 435–459 (2017).
Lee, S. H., Kim, J. H. & Yoon, J. R. Laser scribed graphene cathode for next generation of high performance hybrid supercapacitors. Sci. Rep. 8, 1–9 (2018).
Ju, X. et al. Surfactant-assisted synthesis of high energy {010} facets beneficial to Li-ion transport kinetics with layered LiNi0.6Co0.2Mn0.2O2. ACS Sustain. Chem. Eng. 6, 6312–6320 (2018).
Kim, S. W., Seo, D. H., Ma, X., Ceder, G. & Kang, K. Electrode materials for rechargeable sodium-ion batteries: Potential alternatives to current lithium-ion batteries. Adv. Energy Mater. 2, 710–721 (2012).
Lee, S. H., **, B. S. & Kim, H. S. Superior performances of B-doped LiNi0.84Co0.10Mn0.06O2 cathode for advanced LIBs. Sci. Rep. 9, 1–7 (2019).
Du Pasquier, A., Plitz, I., Menocal, S. & Amatucci, G. A comparative study of Li-ion battery, supercapacitor and nonaqueous asymmetric hybrid devices for automotive applications. J. Power Sources 115, 171–178 (2003).
Purwanto, A. et al. NCA cathode material: Synthesis methods and performance enhancement efforts. Mater. Res. Express 5, 122001 (2018).
Dahn, J. R., Fuller, E. W., Obrovac, M. & von Sacken, U. Thermal stability of LixCoO2, LixNiO2 and λ-MnO2 and consequences for the safety of Li-ion cells. Solid State Ionics 69, 265–270 (1994).
Vu, D. L. & Lee, J. Properties of LiNi0.8Co0.1Mn0.1O2 as a high energy cathode material for lithium-ion batteries. Korean J. Chem. Eng. 33, 514–526 (2016).
Sim, S. J., Lee, S. H., **, B. S. & Kim, H. S. Effects of lithium tungsten oxide coating on LiNi0.90Co0.05Mn0.05O2 cathode material for lithium-ion batteries. J. Power Sources 481, 229037 (2021).
Lee, S. H., Park, G. J., Sim, S. J., **, B. S. & Kim, H. S. Improved electrochemical performances of LiNi0.8Co0.1Mn0.1O2 cathode via SiO2 coating. J. Alloys Compd. 791, 193–199 (2019).
Lee, S. H., Lee, S., **, B. S. & Kim, H. S. Optimized electrochemical performance of Ni rich LiNi0.91Co0.06Mn0.03O2 cathodes for high-energy lithium ion batteries. Sci. Rep. 9, 1–7 (2019).
Sim, S. J., Lee, S. H., **, B. S. & Kim, H. S. Use of carbon coating on LiNi0.8Co0.1Mn0.1O2 cathode material for enhanced performances of lithium-ion batteries. Sci. Rep. 10, 1–9 (2020).
Majumder, S. B., Nieto, S. & Katiyar, R. S. Synthesis and electrochemical properties of LiNi0.80(Co0.20–xAlx)O2 (x = 0.0 and 0.05) cathodes for Li ion rechargeable batteries. J. Power Sources 154, 262–267 (2006).
Kannan, A. M. & Manthiram, A. Structural stability of Li1−xNi0.85Co0.15O2 and Li1−xNi0.85Co0.12Al0.03O2 cathodes at elevated temperatures. J. Electrochem. Soc. 150, A349 (2003).
Fan, Z. Y., **, E. M. & Jeong, S. M. Enhanced electrochemical properties of NCA cathode materials for lithium ion battery by do** effect. Korean Chem. Eng. Res. 55, 861–867 (2017).
Li, J. et al. Facilitating the operation of lithium-ion cells with high-nickel layered oxide cathodes with a small dose of aluminum. Chem. Mater. 30, 3101–3109 (2018).
Jo, M., Noh, M., Oh, P., Kim, Y. & Cho, J. A new high power LiNi0.81Co0.1Al0.09O2 cathode material for lithium-ion batteries. Adv. Energy Mater. 4, 1–8 (2014).
Hou, P., Zhang, H., Deng, X., Xu, X. & Zhang, L. Stabilizing the electrode/electrolyte interface of LiNi0.8Co0.15Al0.05O2 through tailoring aluminum distribution in microspheres as long-life, high-rate, and safe cathode for lithium-ion batteries. ACS Appl. Mater. Interfaces 9, 29643–29653 (2017).
Myung, S. T. et al. Nickel-rich layered cathode materials for automotive lithium-ion batteries: Achievements and perspectives. ACS Energy Lett. 2, 196–223 (2017).
**a, Y., Zheng, J., Wang, C. & Gu, M. Designing principle for Ni-rich cathode materials with high energy density for practical applications. Nano Energy 49, 434–452 (2018).
Qiu, Z., Zhang, Y., Dong, P., **a, S. & Yao, Y. A facile method for synthesis of LiNi0.8Co0.15Al0.05O2 cathode material. Solid State Ionics 307, 73–78 (2017).
Zhang, H., Yang, S., Huang, Y. & Hou, X. Synthesis of non-spherical LiNi0.88Co0.09Al0.03O2 cathode material for lithium-ion batteries. Energy Fuels 34, 9002–9010 (2020).
Li, W., Reimers, J. N. & Dahn, J. R. In situ X-ray diffraction and electrochemical studies of Li1−xNiO2. Solid State Ionics 67, 123–130 (1993).
Park, T. J., Lim, J. B. & Son, J. T. Effect of calcination temperature of size controlled microstructure of LiNi0.8Co0.15Al0.05O2 cathode for rechargeable lithium battery. Bull. Korean Chem. Soc. 35, 357–364 (2014).
Ryu, H. H., Park, G. T., Yoon, C. S. & Sun, Y. K. Microstructural degradation of Ni-rich Li[NixCoyMn1−x−y]O2 Cathodes during accelerated calendar aging. Small 14, 1803179 (2018).
Nara, H. et al. Impedance analysis of LiNi1/3Mn1/3Co1/3O2 cathodes with different secondary-particle size distribution in lithium-ion battery. Electrochim. Acta 241, 323–330 (2017).
Oldenburger, M. et al. Investigation of the low frequency Warburg impedance of Li-ion cells by frequency domain measurements. J. Energy Storage 21, 272–280 (2019).
Matsumoto, K., Kuzuo, R., Takeya, K. & Yamanaka, A. Effects of CO2 in air on Li deintercalation from LiNi1−x−yCoxAlyO2. J. Power Sources 81–82, 558–561 (1999).
Li, Y. K., Wang, W. X., Wu, C. & Yang, J. Screen-printed dual-particle-containing multicomponent-blending carbon nanotube cathode: Enhancement of electron emission characteristics current emission stability, and uniformity. Trans. Electr. Electron. Mater. 23, 64–71 (2022).
Kim, Y., Park, H., Warner, J. H. & Manthiram, A. Unraveling the intricacies of residual lithium in high-Ni cathodes for lithium-ion batteries. ACS Energy Lett. 6, 941–948 (2021).
Acknowledgements
This work was supported by the Development Program [20011379, Development of advanced charge acceptance technology for charging power improvement of xEV battery system] and [20015809, Development of particle shape controlled nickel-based cathode material with high capacity and long cycle life] funded by the Ministry of Trade, Industry and Energy (MOTIE), Korea. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021R1F1A1055979).
Author information
Authors and Affiliations
Contributions
H.J.P. and S.H.L. wrote the main manuscript text. B.S.J. and S.J.S. carried out the fabrication of sample and interpretation of the results. B.S.J. and H.S.K. initiated the idea of working on the present topic. H.J.P. and S.J.S. analyzed all the experiments. All the authors read and approved the final manuscript. All data generated or analysed during this study are included in this published article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, HJ., Sim, SJ., **, BS. et al. Influence of sintering temperatures on microstructure and electrochemical performances of LiNi0.93Co0.04Al0.03O2 cathode for high energy lithium ion batteries. Sci Rep 12, 9617 (2022). https://doi.org/10.1038/s41598-022-13843-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-13843-5
- Springer Nature Limited