Abstract
Peripheral neuropathies result in adaptation in primary sensory and other regions of cortex, and provide a framework for understanding the localized and widespread adaptations that arise from altered sensation. Mesoscale cortical imaging achieves high temporal resolution of activity using optical sensors of neuronal activity to simultaneously image across a wide expanse of cortex and capture this adaptation using sensory-evoked and spontaneous cortical activity. Saphenous nerve ligation in mouse is an animal model of peripheral neuropathy that produces hyperalgesia circumscribed to the hindlimb. We performed saphenous nerve ligation or sham, followed by mesoscale cortical imaging using voltage sensitive dye (VSD) after ten days. We utilized subcutaneous electrical stimulation at multiple stimulus intensities to characterize sensory responses after ligation or sham, and acquired spontaneous activity to characterize functional connectivity and large scale cortical network reorganization. Relative to sham animals, the primary sensory-evoked response to hindlimb stimulation in ligated animals was unaffected in magnitude at all stimulus intensities. However, we observed a diminished propagating wave of cortical activity at lower stimulus intensities in ligated animals after hindlimb, but not forelimb, sensory stimulation. We simultaneously observed a widespread decrease in cortical functional connectivity, where midline association regions appeared most affected. These results are consistent with localized and broad alterations in intracortical connections in response to a peripheral insult, with implications for novel circuit level understanding and intervention for peripheral neuropathies and other conditions affecting sensation.
Similar content being viewed by others
Introduction
Peripheral neuropathies can result in hyperalgesia and allodynia, and are thus a major cause of chronic pain1. These localized peripheral insults cause a range of peripheral and central nervous system adaptations that ultimately result in significant direct and indirect health costs2.
Peripheral neuropathies cause alterations to sensory circuits inducing adaptations in peripheral nociceptive fibres, descending control system, and excitatory transmission in the spinal cord dorsal horn3,4,5,6,7. Recently, reorganization of higher order sensory and affective processing regions has been observed, leading to a focus on understanding changes occurring in higher CNS regions and how these alterations might contribute to the persistence of altered nociception and its broader repercussions8,9,10.
Cortical sensory processing in the absence of pathology involves thalamocortical inputs to primary somatosensory cortex, and from this primary sensory-evoked response propagates a wave of activity that spreads via horizontal connections beyond the primary response region11,12. Though the function of these waves is unclear, several roles have been proposed including temporal information relating to stimulus onset and multisensory integration12. These long-range intracortical connections indicate recurrent interactions within cortex13, and we hypothesized that they would be susceptible to pathological adaptations and associated with changes in spontaneous activity dynamics in which they participate.
Experimental chronic peripheral neuropathy14,15,16,17,18 perturbs sensory circuits by selectively inducing aberrant sensory information to a localized region of somatosensory cortex and produces a behavioral phenotype of hyperalgesia similar to clinical neuropathic pain. Synaptic changes occur rapidly in rodent models of neuropathy19,20, with stabilization and sustained changes reflected in large scale brain network changes21. Using the saphenous nerve ligation model together with millisecond resolution voltage sensitive dye (VSD) cortical imaging over a large expanse of dorsal neocortex in mouse, we characterize sensory responses and their associated propagating waves.
To further characterize putative network adaptations after peripheral neuropathy, we leverage functional connectivity analysis in spontaneous activity as these interregional activity changes also represent recurrent interactions within cortex and moreover align with human fMRI findings. In human studies, alterations in the structure of spontaneous brain activity associated with allodynia are partially overlap** yet unique from the pain networks activated in response to evoked stimuli22,23,24. The observed alterations are centered on the default mode network (DMN), of which midline regions of cortex including the cingulate and medial prefrontal cortex in particular showing decreased connectivity to other nodes in the DMN. This reorganization is thought to accompany the development of chronic pain25,26. Emerging fMRI data in mouse suggests that midline cortical regions consistent with the murine DMN27 demonstrate altered functional connectivity after peripheral neuropathy21. Here, we build on this literature by leveraging the temporal resolution of VSD and a wide field of view to examine alterations in functional connectivity.
Results
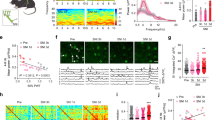
A large bilateral craniotomy exposed numerous cortical regions, including primary and secondary somatosensory and motor regions of the hindlimb, forelimb, and whisker, along with the anterior and midline association cortices: anterior cingulate (aM2/AC), secondary motor (pM2), retrosplenial (RS), and parietal association (PtA) cortices. (Fig. 1a). Hindlimb (Fig. 1bi) and Forelimb (Fig. 1ci) sensory stimulation via electrical stimulation produced a characteristic voltage response that originated in the primary somatosensory region in the hemisphere contralateral to the stimulated limb. Over tens of milliseconds, the voltage response was observed propagating outward from the primary region, along with a secondary voltage response in the primary somatosensory region of the ipsilateral hemisphere. Optical flow (represented by vector arrows in Fig. 1bii,cii) captures the propagation of voltage fluctuations as they spread over the cortex. It is important to note that localized voltage fluctuations centered around a maxima without generating a propagating wave are not associated with such vectors.
Voltage sensitive dye mesoscale cortical imaging. (a) A representative image of the exposed cortex after bilateral craniotomy and dura removal. At left the schematic represents cortical topography of the regions of interest. (bi) A representative time-course of the voltage responses, measured as a percentage change in fluorescence relative to baseline in a sham animal. Hindlimb stimulation results in a primary response in the contralateral hemisphere that spreads across the cortical surface, with a secondary response in the ipsilateral hemisphere. (bii) A representative time-course of optical flow, as measured by the frame-to-frame change accounted for by virtual pixel movement. Both the primary response and the secondary response elicit outward moving optical flow. Arrows represent relative magnitude and direction of optical flow. Each optical flow figure represents the forward-looking change between the above frame and the subsequent frame. (ci) Forelimb stimulation results in a primary response in the contralateral hemisphere that spreads across the cortical surface, with a secondary response in the ipsilateral hemisphere. (cii) As in hindlimb stimulation, propagation of the primary response results in transient optical flow outwards from the primary somatosensory region.
Saphenous nerve ligation (Fig. 2a) produced sustained hyperalgesia as assessed by mechanical withdrawal threshold to von Frey filaments applied to affected limb relative to sham. While withdrawal thresholds were similar at baseline, following surgery ligated animals (n = 8) showed a significant sensitization of withdrawal relative to (n = 6) sham (two-way RM ANOVA significant group effect, F(1,12) = 6.885, p = 0.022, and effect of day F(4,12) = 8.659, p < 0.001, significant interaction F(4,12) = 7.484, p < 0.001, follow-up pairwise comparisons p < 0.01 on days 3, 5, and 7; Fig. 2b). Rodent models of depression can produce broad alterations in cortical connectivity32, however we attempted to mitigate this potential confound by normalizing response and propagating wave magnitude within subjects to a ceiling stimulation intensity (2 mA). With respect to resting state connectivity, conserved structure of spontaneous activity is observable in quiet wakefulness, sleep, and anaesthesia56,57, including using mesoscale cortical VSD imaging32,35,58. Finally, this acute preparation prevented us from repeated sampling to quantify change over time, however novel genetically encoded voltage indicators may permit such approaches in the future59.
Succinctly, we find several indices of cortical reorganization after peripheral neuropathy that transcend primary sensory regions and influence sensory-evoked activity. Identifying the central nodes of this reorganization is a crucial next step which could lead to novel therapeutic strategies based on non-invasive neurostimulation60. In addition, our observation of altered spontaneous connectivity within the slow power band suggest a novel biomarker for neuropathic pain that could aid in diagnosis and guide treatment strategies61.
Methods
Animals
Adult male C57BL6J mice (N = 14, 3–4 months old) were used for all experiments. Animals were group housed on a 12:12 light cycle with ad libitum access to food and water. All procedures were approved by the University of British Columbia Animal Care Committee (UBC ACC) in accordance with the ethical standards set forth by the Canadian Council on Animal Care (CCAC).
Saphenous nerve ligation
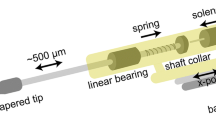
To model focal neuropathic pain, the saphenous nerve of the right hindlimb was ligated ten days prior to imaging14. Mice were anesthetized with isoflurane (5% induction, 2% maintenance in 1 L/min O2) and administered buprenorphine (SC, 0.05 ml, 0.3 mg/ml) for analgesia. Body temperature was maintained at 37 °C with a thermometer feedback controlled heatpad. The anterior aspect of the left hindlimb was shaved and disinfected with betadine and alcohol (3x alternating), and an 8 mm incision was made overlying the saphenous triad. Subcutaneous and connective tissue was blunt dissected to isolate the saphenous nerve and 8-0 braided suture (Vicryl, Ethicon Inc., USA) was tied loosely around the nerve at 1 mm intervals. Sham animals underwent the same incision and dissection surgical procedure but no ligation was performed. Subsequent to ligation, the primary incision was closed (5-0 Vicryl, Ethicon Inc., USA) and mice were recovered and returned to their homecage.
Mechanical withdrawal assay
Mechanical withdrawal threshold was assayed at several points using von Frey Filaments62. Mice were restrained within a modified restraint tube with a metallic mesh base that allowed Von Frey filament application to the hindpaw. To determine the mechanical threshold, von Frey filaments ranging from 0.5 g to 6 g were applied to the plantar surface of the hindpaw. Beginning with the weakest filament, filaments were applied three to five times for two seconds. The animal’s response to filament application was observed, with a positive response defined as withdrawing, lifting, holding, or shaking the paw. The mechanical pain threshold was defined as ≥3 out of 5 positive applications. For subsequent assessments, withdrawal threshold was assessed using an up-down assay at the withdrawal threshold determined in the previous assessment for each animal.
Forced swim test (FST)
Mice were placed in a transparent glass beaker (25 cm height, 18 cm diameter), containing water at 24-5 °C. For 5 minutes, the mice remained in the water while an observer coded their active swimming and floating (including efforts to maintain position). The water was changed between animals. Only the final 4 minutes of the experiment are reported.
VSD Imaging surgery
Ten days after nerve ligation, mice were anesthetized with isoflurane (5% induction, 1.5% maintenance) and prepared for bilateral cortical VSD imaging as previously detailed58,63. Recording of experimental groups was interleaved. Briefly, a screw was fastened to the exposed skull with cyanoacrylate and dental cement for subsequent head fixation, and an approximately 7 × 8 mm bilateral craniotomy was performed to expose the cortical surface 3.5 mm anterior to bregma to 4.5 mm posterior to bregma and 4 mm lateral to bregma. The underlying dura was removed to facilitate dye penetration and enhance optical clarity. RH1692 (Optical Imaging, New York, NY) was dissolved in HEPES buffered saline (1 mg/ml) and applied to the cortex for 60–90 minutes. Following washing of unbound dye with HEPES buffered saline, the brain was covered with 1.5% agarose and sealed with a glass coverslip to minimize respiration artifacts. This preparation reveals a wide expanse of neocortex including sensory, motor, and midline association regions (Fig. 1a). Dye incubation results in penetration throughout all neocortical layers64.
VSD imaging protocol
Mice were head fixed for imaging under light anesthesia (1% isoflurane) with body temperature maintained at 37 °C with a feedback thermistor. 12-bit images were captured with 67um/pixel resolution at 50 Hz for spontaneous activity or 150 Hz for sensory evoked activity with a CCD camera (1M60 Pantera, Dalsa, Waterloo, ON) and EPIX E4DB frame grabber using XCAP 3.8 imaging software (EPIX, Inc., Buffalo Grove, IL) through a macroscope composed of front-to-front video lenses (8.6 × 8.6 mm FOV, 67 µm/pixel). VSD was excited with a red LED (Luxeon K2, 627 nm) filtered at 630 nm +−15 nm and fluorescence captured using a 673–703 nm bandpass optical filter (Semrock, New York, NY). Imaging was focused into the cortex to a depth of ~1 mm to reduce VSD signal distortion from large blood vessels. Ambient light resulting from VSD excitation was measured at 8.65e-3 W/m2. With dye incubation throughout all cortical layers, and diffraction of excitation and emission signals, the bulk of the fluorescent signal originates from neuropil in superficial layers of the cortex64. Spontaneous activity was sampled at 50 Hz for 30 minutes (90,000 frames). For anesthetic free imaging, mice were recovered with oxygen (1 L/min) while head-fixed for 15 minutes, and awake head-fixed spontaneous activity was recorded at 150 Hz for 5.5 minutes (50,000 frames).
Sensory evoked responses
Sensory evoked responses were imaged at 150 Hz for 108 frames (720 ms) in response to forelimb and hindlimb stimulation under light anesthesia (1% isoflurane). For stimulation, 0.14 mm acupuncture needles were inserted into the left paws and 0.5, 1 and 2 mA square pulses (1 ms) were delivered. For both modalities, 10 stimulations in addition to 5 no-stimulation trials were delivered, with an interstimulus interval of 10 s.
Data analysis
VSD image stacks were analyzed using custom-written MATLAB code (Mathworks, MA). For sensory evoked activity, 10 trials were averaged to minimize the effects of spontaneous fluctuations and normalized to a 5 trial average of blank trials. Photobleaching artifacts were removed by using a detrending filter based on the cross pixel time-varying average signal and the per-pixel average fluorescence across the trial. All individual pixel signals were then expressed as a relative change (ΔF/F × 100%) and normalized to the pre-stimulus baseline. A 5 × 5 pixel ROI was selected based on the centroid of peak response for each sensory modality, and secondary sensory modality and association area ROIs were interpolated based on these sensory ROIs and standard stereotaxic coordinates. For spontaneous activity analysis, per-pixel fluorescence fluctuations were first filtered in the slow activity band (0.5–6 Hz) and then expressed as relative change (ΔF/F × 100%).
Optical flow analysis
Optical flow was calculated using the combined local-global (CLG) algorithm of optical flow estimation from the Optical Flow Analysis Toolbox33, producing a per-pixel estimation of the optical flow between image frames. In order to capture broad alterations in optical flow irrespective of direction, the magnitude of optical flow vectors from every pixel in each hemisphere was averaged at each time point, producing a time-varying signal that peaked shortly after sensory stimulation, which we refer to as aggregate optical flow. Optical flow magnitude was integrated over the 10 frames following stimulation (66 ms) to produce a single estimate of optical flow magnitude (integrating across the entire trial did not alter results).
The peak optical flow magnitude was found per pixel for each hemisphere to examine alterations in peak optical flow. A cumulative distribution of peak pixel magnitudes was created for each animal and averaged across animals for each stimulation magnitude.
To test whether hemisphere-wide alterations in normalized optical flow were driven by regionally specific alterations, per-pixel responses were segregated into a primary response region and a surround region. Regions were defined based on peak phase latency relative to stimulus onset, as in the analysis detailed below. The primary region was defined as contiguous pixels whose peak df/F value corresponded to the peak within the 5 × 5 pixel ROI of the primary response, while the remaining pixels were assigned to the surround region. Optical flow magnitudes within these defined regions were averaged for each frame and integrated across 10 frames as above.
Peak phase latency analysis
The temporal offset of peak activity was used as an alternative analysis of propagating activity34. Processed evoked ΔF/F was additionally filtered between 5 and 30 Hz on a per pixel basis, and the first local peak of activity after stimulation was found for each pixel. Contiguous areas with simultaneous peak latency were identified by a one step erosion/dilation for each latency followed by removal of minimally sized regions with peak responses at each frame. This resulted in typically one or two large contiguous regions, however any remaining regions were included in analysis, which was confined to the contralateral (primary) hemisphere. Total area was calculated by a sum of participating pixels, while average response magnitude was calculated from the mean df/F across pixels for each frame.
Functional connectivity analysis
Correlation matrices were created based on the zero-lag Pearson correlation of ROI pairs filtered in the slow activity band (0.5–6 Hz) during 30 minutes of spontaneous activity acquired at 50 Hz filtered or 5.5 minutes of spontaneous activity in a period of quiet wakefulness. Individual ROIs were calculated for all animals based on interpolation from the peak responses to hindlimb, forelimb, and whisker sensory responses. To visualize network changes, average differences were plotted as an undirected network diagram using modified code from the Bioinformatics and Brain Connectivity Toolbox65. To facilitate visualization, each connection was only represented if the correlation value was 10% greater or inferior to that observed in the sham group. To test the statistical significance of functional connectivity differences between groups, we utilized generalized linear mixed effect models (GLMEM; Wilkinson notation: Inter-Regional_Correlation ~1 + Group + (1|Region) + (1|Mouse)) implemented in MATLAB.
Data availability
The datasets generated and analysed for the current study are available from the corresponding author on reasonable request.
References
Breivik, H., Collett, B., Ventafridda, V., Cohen, R. & Gallacher, D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. European Journal of Pain 10, 287–287 (2006).
Okie, S. A flood of opioids, a rising tide of deaths. N. Engl. J. Med. 363, 1981–1985 (2010).
Campbell, J. N. & Meyer, R. A. Mechanisms of neuropathic pain. Neuron 52, 77–92 (2006).
Sandkühler, J. Models and Mechanisms of Hyperalgesia and Allodynia. Physiological Reviews 89, 707–758 (2009).
Basbaum, A. I., Bautista, D. M., Scherrer, G. & Julius, D. Cellular and Molecular Mechanisms of Pain. Cell 139, 267–284 (2009).
Kuner, R. Central mechanisms of pathological pain. Nature Medicine 16, 1258–1266 (2010).
Colloca, L. et al. Neuropathic pain. Nat Rev Dis Primers 3, 17002 (2017).
Kuner, R. & Flor, H. Structural plasticity and reorganisation in chronic pain. Nature Reviews Neuroscience 18, 20–30 (2017).
Bushnell, M. C., Čeko, M. & Low, L. A. Cognitive and emotional control of pain and its disruption in chronic pain. Nature Reviews Neuroscience 14, 502–511 (2013).
Bliss, T. V. P., Collingridge, G. L., Kaang, B.-K. & Zhuo, M. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nature Reviews Neuroscience 17, 485–496 (2016).
Sato, T. K., Nauhaus, I. & Carandini, M. Traveling waves in visual cortex. Neuron 75, 218–229 (2012).
Muller, L., Chavane, F., Reynolds, J. & Sejnowski, T. J. Cortical travelling waves: mechanisms and computational principles. Nat. Rev. Neurosci. 19, 255–268 (2018).
Wu, J.-Y., Huang, X. & Zhang, C. Propagating waves of activity in the neocortex: what they are, what they do. Neuroscientist 14, 487–502 (2008).
Walczak, J.-S., Pichette, V., Leblond, F., Desbiens, K. & Beaulieu, P. Characterization of chronic constriction of the saphenous nerve, a model of neuropathic pain in mice showing rapid molecular and electrophysiological changes. J. Neurosci. Res. 83, 1310–1322 (2006).
Gunduz, O., Oltulu, C., Guven, R., Buldum, D. & Ulugol, A. Pharmacological and behavioral characterization of the saphenous chronic constriction injury model of neuropathic pain in rats. Neurological Sciences 32, 1135–1142 (2011).
Walczak, J.-S. et al. Behavioral, pharmacological and molecular characterization of the saphenous nerve partial ligation: A new model of neuropathic pain. Neuroscience 132, 1093–1102 (2005).
Decosterd, I. & Woolf, C. J. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 87, 149–158 (2000).
Seltzer, Z., Dubner, R. & Shir, Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain 43, 205–218 (1990).
Kim, S. K. & Nabekura, J. Rapid synaptic remodeling in the adult somatosensory cortex following peripheral nerve injury and its association with neuropathic pain. J. Neurosci. 31, 5477–5482 (2011).
Metz, A. E., Yau, H.-J., Centeno, M. V., Apkarian, A. V. & Martina, M. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc. Natl. Acad. Sci. USA 106, 2423–2428 (2009).
Komaki, Y. et al. Functional brain map** using specific sensory-circuit stimulation and a theoretical graph network analysis in mice with neuropathic allodynia. Scientific Reports 6 (2016).
Baliki, M. N., Mansour, A. R., Baria, A. T. & Apkarian, A. V. Functional reorganization of the default mode network across chronic pain conditions. PLoS One 9, e106133 (2014).
Baliki, M. N. et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J. Neurosci. 26, 12165–12173 (2006).
Baliki, M. N., Geha, P. Y., Apkarian, A. V. & Chialvo, D. R. Beyond Feeling: Chronic Pain Hurts the Brain, Disrupting the Default-Mode Network Dynamics. Journal of Neuroscience 28, 1398–1403 (2008).
Apkarian, A. V., Hashmi, J. A. & Baliki, M. N. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain 152, S49–64 (2011).
Malinen, S. et al. Aberrant temporal and spatial brain activity during rest in patients with chronic pain. Proc. Natl. Acad. Sci. USA 107, 6493–6497 (2010).
Lu, H. et al. Rat brains also have a default mode network. Proceedings of the National Academy of Sciences 109, 3979–3984 (2012).
McGirr, A., LeDue, J., Chan, A. W., **e, Y. & Murphy, T. H. Cortical functional hyperconnectivity in a mouse model of depression and selective network effects of ketamine. Brain 140, 2210–2225 (2017).
Petit-Demouliere, B., Chenu, F. & Bourin, M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology 177, 245–255 (2005).
Inouye, T., Shinosaki, K., Iyama, A., Matsumoto, Y. & Toi, S. Moving potential field of frontal midline theta activity during a mental task. Brain Res. Cogn. Brain Res. 2, 87–92 (1994).
Townsend, R. G. et al. Emergence of complex wave patterns in primate cerebral cortex. J. Neurosci. 35, 4657–4662 (2015).
Mohajerani, M. H. et al. Spontaneous cortical activity alternates between motifs defined by regional axonal projections. Nat. Neurosci. 16, 1426–1435 (2013).
Afrashteh, N., Inayat, S., Mohsenvand, M. & Mohajerani, M. H. Optical-flow analysis toolbox for characterization of spatiotemporal dynamics in mesoscale optical imaging of brain activity. Neuroimage 153, 58–74 (2017).
Muller, L., Reynaud, A., Chavane, F. & Destexhe, A. The stimulus-evoked population response in visual cortex of awake monkey is a propagating wave. Nat. Commun. 5, 3675 (2014).
Mohajerani, M. H., McVea, D. A., Fingas, M. & Murphy, T. H. Mirrored bilateral slow-wave cortical activity within local circuits revealed by fast bihemispheric voltage-sensitive dye imaging in anesthetized and awake mice. J. Neurosci. 30, 3745–3751 (2010).
Large, A. M., Vogler, N. W., Mielo, S. & Oswald, A.-M. M. Balanced feedforward inhibition and dominant recurrent inhibition in olfactory cortex. Proc. Natl. Acad. Sci. USA 113, 2276–2281 (2016).
Zhang, Z. et al. Role of Prelimbic GABAergic Circuits in Sensory and Emotional Aspects of Neuropathic Pain. Cell Rep. 12, 752–759 (2015).
Cha, M. H. et al. Modification of cortical excitability in neuropathic rats: a voltage-sensitive dye study. Neurosci. Lett. 464, 117–121 (2009).
Eto, K. et al. Inter-regional contribution of enhanced activity of the primary somatosensory cortex to the anterior cingulate cortex accelerates chronic pain behavior. J. Neurosci. 31, 7631–7636 (2011).
Pinheiro, E. S. S. et al. Electroencephalographic Patterns in Chronic Pain: A Systematic Review of the Literature. PLoS One 11, e0149085 (2016).
Baron, R., Baron, Y., Disbrow, E. & Roberts, T. P. Brain processing of capsaicin-induced secondary hyperalgesia: a functional MRI study. Neurology 53, 548–557 (1999).
Maihöfner, C., Schmelz, M., Forster, C., Neundörfer, B. & Handwerker, H. O. Neural activation during experimental allodynia: a functional magnetic resonance imaging study. Eur. J. Neurosci. 19, 3211–3218 (2004).
Wager, T. D. et al. An fMRI-Based Neurologic Signature of Physical Pain. New England Journal of Medicine 368, 1388–1397 (2013).
Xu, H. et al. Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. J. Neurosci. 28, 7445–7453 (2008).
Ning, L., Ma, L.-Q., Wang, Z.-R. & Wang, Y.-W. Chronic constriction injury induced long-term changes in spontaneous membrane-potential oscillations in anterior cingulate cortical neurons in vivo. Pain Physician 16, E577–89 (2013).
Blom, S. M., Pfister, J.-P., Santello, M., Senn, W. & Nevian, T. Nerve injury-induced neuropathic pain causes disinhibition of the anterior cingulate cortex. J. Neurosci. 34, 5754–5764 (2014).
Stafford, J. M. et al. Large-scale topology and the default mode network in the mouse connectome. Proc. Natl. Acad. Sci. USA 111, 18745–18750 (2014).
Baliki, M. N., Baria, A. T. & Apkarian, A. V. The Cortical Rhythms of Chronic Back Pain. Journal of Neuroscience 31, 13981–13990 (2011).
Kucyi, A. et al. Enhanced medial prefrontal-default mode network functional connectivity in chronic pain and its association with pain rumination. J. Neurosci. 34, 3969–3975 (2014).
Napadow, V. et al. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 62, 2545–2555 (2010).
Loggia, M. L. et al. Default mode network connectivity encodes clinical pain: an arterial spin labeling study. Pain 154, 24–33 (2013).
Hemington, K. S., Wu, Q., Kucyi, A., Inman, R. D. & Davis, K. D. Abnormal cross-network functional connectivity in chronic pain and its association with clinical symptoms. Brain Struct. Funct. 221, 4203–4219 (2016).
Alshelh, Z. et al. Chronic Neuropathic Pain: It’s about the Rhythm. J. Neurosci. 36, 1008–1018 (2016).
Bair, M. J., Robinson, R. L., Katon, W. & Kroenke, K. Depression and pain comorbidity: a literature review. Arch. Intern. Med. 163, 2433–2445 (2003).
Kaiser, R. H., Andrews-Hanna, J. R., Wager, T. D. & Pizzagalli, D. A. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry 72, 603–611 (2015).
Vincent, J. L. et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447, 83–86 (2007).
Horovitz, S. G. et al. Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG-fMRI study. Hum. Brain Mapp. 29, 671–682 (2008).
Chan, A. W., Mohajerani, M. H., LeDue, J. M., Wang, Y. T. & Murphy, T. H. Mesoscale infraslow spontaneous membrane potential fluctuations recapitulate high-frequency activity cortical motifs. Nat. Commun. 6, 7738 (2015).
Piatkevich, K. D. et al. A robotic multidimensional directed evolution approach applied to fluorescent voltage reporters. Nat. Chem. Biol. 14, 352–360 (2018).
Leung, A. et al. rTMS for suppressing neuropathic pain: a meta-analysis. J. Pain 10, 1205–1216 (2009).
Kucyi, A. & Davis, K. D. The Neural Code for Pain: From Single-Cell Electrophysiology to the Dynamic Pain Connectome. Neuroscientist 23, 397–414 (2017).
Sousa, M. V. Pde et al. Building, testing and validating a set of home-made von Frey filaments: A precise, accurate and cost effective alternative for nociception assessment. Journal of Neuroscience Methods 232, 1–5 (2014).
Zhang, S. & Murphy, T. H. Imaging the impact of cortical microcirculation on synaptic structure and sensory-evoked hemodynamic responses in vivo. PLoS Biol. 5, e119 (2007).
Grinvald, A. & Hildesheim, R. VSDI: a new era in functional imaging of cortical dynamics. Nat. Rev. Neurosci. 5, 874–885 (2004).
Rubinov, M. & Sporns, O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52, 1059–1069 (2010).
Author information
Authors and Affiliations
Contributions
A.M. and T.H.M. conceived of the study. A.M. performed all experiments and collected the data. D.M.A., J.L. and A.M. analyzed the data. D.M.A. and A.M. wrote the manuscript. All authors reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ashby, D.M., LeDue, J., Murphy, T.H. et al. Peripheral Nerve Ligation Elicits Widespread Alterations in Cortical Sensory Evoked and Spontaneous Activity. Sci Rep 9, 15341 (2019). https://doi.org/10.1038/s41598-019-51811-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-51811-8
- Springer Nature Limited
This article is cited by
-
Mesoscopic cortical network reorganization during recovery of optic nerve injury in GCaMP6s mice
Scientific Reports (2020)