Abstract
Cognitive dysfunction (CD) in heart failure (HF) adversely affects treatment compliance and quality of life. Although ryanodine receptor type 2 (RyR2) has been linked to cardiac muscle dysfunction, its role in CD in HF remains unclear. Here, we show in hippocampal neurons from individuals and mice with HF that the RyR2/intracellular Ca2+ release channels were subjected to post-translational modification (PTM) and were leaky. RyR2 PTM included protein kinase A phosphorylation, oxidation, nitrosylation and depletion of the stabilizing subunit calstabin2. RyR2 PTM was caused by hyper-adrenergic signaling and activation of the transforming growth factor-beta pathway. HF mice treated with a RyR2 stabilizer drug (S107), beta blocker (propranolol) or transforming growth factor-beta inhibitor (SD-208), or genetically engineered mice resistant to RyR2 Ca2+ leak (RyR2-p.Ser2808Ala), were protected against HF-induced CD. Taken together, we propose that HF is a systemic illness driven by intracellular Ca2+ leak that includes cardiogenic dementia.
Similar content being viewed by others
Main
HF is the most rapidly growing cardiovascular disorder affecting millions worldwide1,2, with associated high rates of mortality, poor quality of life, and high health care costs due to decreased cardiac function and dysfunction of other organ systems3,4,5. Recent studies suggest that CD in HF, known as ‘cardiogenic dementia’ may be caused by HF itself, with a prevalence of 20–80%6,7.
CD includes forgetfulness and poor learning ability, which may impair self-care8,9,10 and compliance11,12 in as many as 90% of those with HF. Noncompliance increases the risk of mortality and morbidity13. Indeed, CD impairs the ability of individuals with HF to make decisions in critical situations, such as early recognition and interpretation of worsening symptoms, and making appropriate decisions about their health. People with HF and preserved ejection fraction also exhibit CD14, including verbal memory and executive function deficits, known as cognitive inflexibility15. Structural changes in the brain including atrophy, increased white matter hyper-intensities, gray matter loss and silent cerebral infarction, are frequently observed in HF patients with CD16,17. Interestingly, these structural and functional changes coincide with a chronic inflammatory response and neurohormonal activation including the renin–angiotensin–aldosterone system and the adrenergic pathway18. Furthermore, clinical studies have linked cardiovascular diseases, dementia and Alzheimer’s disease through common triggers, including inflammation, oxidative stress, hypoxia19 and adrenergic signaling20,21,22.
Indeed, norepinephrine modulates the levels of consciousness23,24. The sympathetic nervous system is continuously activated in patients with HF25 and is known to be part of a major upstream signaling pathway that alters intracellular Ca2+ homeostasis and tightly controls neuronal cellular function and survival. Ca2+ dyshomeostasis is a hallmark of neurodegenerative diseases, including Alzheimer’s disease26, Huntington’s disease27 and Parkinson’s disease28. Intracellular Ca2+ signaling plays a role in regulating long-term potentiation (LTP), long-term depression and neurodegeneration29,30,31.
In neurons, activation of inositol-1,4,5-trisphosphate receptors (IP3Rs) and RyRs amplifies intracellular Ca2+ signals32. Increased intracellular Ca2+ concentration activates Ca2+-dependent processes involved in plasticity and synaptic transmission that are required for learning and memory33. RyR2, the Ca2+-activated intracellular Ca2+ release channel on the sarcoplasmic reticulum (SR) or endoplasmic reticulum (ER), is a homotetrameric macromolecular protein complex that includes four RyR2 monomers, 565-kDa polypeptide each34. The RyR2 channel is regulated by kinases and phosphatases35, phosphodiesterase36, calmodulin37, and the stabilizing subunit calstabin2 (FKBP12.6)35. Protein kinase A (PKA) and Ca2+/calmodulin-dependent protein kinase II (CAMKII) tether to RyR2 and phosphorylate the channel at Ser2808 and Ser2814, respectively35,38. PKA hyper-phosphorylation and/or oxidation/nitrosylation of RyR2 cause calstabin2 dissociation, leading to leaky channels that do not close properly35,39.
We40 and others have previously reported that RyR channels are dysfunctional not only in the cardiomyocytes of patients with HF35,41 but also in the skeletal muscle42,43, suggesting the existence of a common mechanism that primarily affects RyR2 in the cardiac muscle and propagates to affect RyRs in other organs expressing different isoforms of the channels, such as RyR2 in the pancreatic beta cells44 (may cause diabetes) and in the brain (may impair cognitive function), and RyR1 in the diaphragm/lung (may cause respiratory disorders) and locomotor muscle (may cause exercise intolerance and muscle fatigue).
In this study, we found that the hyper-adrenergic state and the enhanced inflammatory response in HF caused neuronal RyR2-mediated intracellular Ca2+ leak that subsequently affected cognition and memory. Neuronal Ca2+ dyshomeostasis increased mitochondrial Ca2+ content, contributing to oxidative overload, and altered the expression of key genes involved in cognitive function. Stabilizing leaky RyR2 channels using a small-molecule Rycal drug S107 prevented cognitive impairment induced by HF.
Results
Neuronal RyR2 channels are leaky in individuals with heart failure
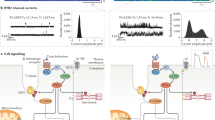
To evaluate RyR2 in the brains of individuals with HF, hippocampal biopsy samples from controls (non-HF) and de-identified individuals with HF (Supplementary Tables 1 and 2) were obtained from the Brain Bank at Columbia University and the National Institutes of Health (NIH) Neuro-Biobank. Immunoprecipitated RyR2 and isolated ER fractions were used to analyze the composition of the hippocampal RyR2 macromolecular complex and PTMs known to be associated with RyR channel Ca2+ leak31,32,35. Hippocampal RyR2 from individuals with HF (n = 9) exhibited PKA hyper-phosphorylation (on Ser2808), oxidation, cysteine nitrosylation, and were depleted of calstabin2, compared to controls (n = 4; Fig. 1a,b). This is the ‘biochemical signature’ of ‘leaky’ RyR2 channels35,45. Single-channel recordings of hippocampal RyR2, reconstituted into planar lipid bilayers, revealed increased open probability (Po), increased mean open time (To) and decreased mean closed time (Tc) in individuals with HF compared to controls (Po = 0.19% ± 0.02%, To = 18 ± 2 ms, Tc = 58 ± 05 ms in HF hippocampi, n = 9; versus Po = 0.01% ± 0.002%, To = 2 ± 0.2 ms, Tc = 515 ± 52 ms in controls, n = 4; P < 0.05) in the presence of low, non-activating [Ca2+]cis (150 nM), conditions under which normal RyR2 channels are tightly closed (Fig. 1c,d). This elevated Po is consistent with pathological hippocampal ER Ca2+ leak26,31. Indeed, neuronal microsomes from individuals with HF exhibited increased RyR-mediated ER Ca2+ leak compared with controls (Fig. 1e,f).
a,b, Representative SDS–PAGE analysis and quantification of modified RyR2 and calstabin2 immunoprecipitated from hippocampi of controls and individuals with HF (IP RyR2; bands normalized to total RyR2). Control (CTRL), n = 4); HF, n = 9. c, Single-channel recordings of RyR2 incorporated in planar lipid bilayers with 150 nM Ca2+ in the cis chamber, corresponding to representative experiments performed using human hippocampal samples from controls and HF patients (two traces from two different controls and individuals with HF are shown) d, Po, To and Tc of RyR2 channels in controls (n = 5) and HF (n = 9) hippocampi. e, ER Ca2+ leak measured in microsomes from control (n = 4) and HF participant (n = 9) hippocampi. f, Bar graphs represent the quantification of microsomal Ca2+ leak as the percentage of uptake in controls (n = 5) and HF individuals (n = 9). Individual values are shown with the mean ± s.e.m. (t-test *P < 0.05, controls versus HF individuals). Data are derived from biologically independent samples. All statistical tests were two sided. a.u., arbitrary units.
Impaired cognitive function in mouse model of heart failure
Because of the complexity of the clinical manifestation of HF patients, we used the mouse model of HF (myocardial infarction, MI) with reduced ejection fraction83 due to defective Ca2+ regulation and inflammation. HF patients and MI mice exhibited increased TGF-β and SMAD3 phosphorylation levels that potentially play a role in cardiogenic dementia.
Increased adrenergic activity and inflammatory pathway activation in HF primarily impairs intracellular Ca2+ regulation. Excessive ER Ca2+ leak enhances oxidative stress, dysregulates neuronal gene/protein expression and primes neurodegenerative pathways. These subcellular changes impair the learning and memory processes in HF, which is detrimental for patients’ compliance to medication and early recognition of worsening symptoms. These pathways are summarized in Fig. 8.
Increased catecholamine levels during HF activate PKA, which phosphorylates RyR2 on Ser2808 (Fig. 3). Increased inflammation in HF includes activation of the TGF-β pathway resulting in SMAD3 phosphorylation and upregulation of NOX2 and binding to RyR2 (Extended Data Fig. 4). NOX2 promotes oxidation of RyR2 channels58,59,60. The combination of oxidation and phosphorylation of RyR2 results in ER Ca2+ leak (Fig. 3). Ca2+ leak through RyR2 leads to increased mitochondrial Ca2+ accumulation, which enhances mitochondrial ROS production (Extended Data Fig. 10). Therefore, a vicious cycle is created between the mitochondria and RyR2, where increased ER Ca2+ leak causes mitochondrial ROS production and increased mitochondrial ROS production further oxidizes RyR2 and renders it leakier. Chronic RyR2 Ca2+ leak depletes ER Ca2+ content and reduces the Ca2+ transient (Fig. 5) required for synaptic vesicle release during synaptic transmission (Figs. 4 and 7). Furthermore, oxidative stress and Ca2+ dyshomeostasis alter gene transcription (Extended Data Fig. 7), with a particular effect on proteins that are regulated by Ca2+ and involved in neurotransmission. Dysregulation of key proteins involved in synaptic transmission is reflected in the impaired LTP observed in the MI mice (Fig. 4b,c). Accumulation of Ca2+ in the cytosol activates Ca2+-dependent enzymes including CAMKII, GSK-β, CDK5 and p25, which subsequently leads to Tau phosphorylation, a hallmark of neurodegenerative disease (Supplementary Figs. 9 and 10). All these activated signaling cascades can be prevented, at least in part, by S107, a Rycal drug that reduces the ER Ca2+ leak. Gs, G protein; AC, adenylyl cyclase; cAMP, cyclic AMP; GSK-β, glycogen synthase kinase 3 beta. Created with BioRender.com.
Limitations of our study include a small sample size of only nine individuals with HF who had incomplete clinical information. We used multiple mouse models to overcome these limitations. Moreover, all individuals with HF were younger than controls, minimizing age-related confounding factors. Other factors such as individuals’ backgrounds, socioeconomic status, hospitalization history and drug use could, however, contribute to CD.
Methods
Human samples
De-identified human hippocampus and cortex samples were obtained from the Brain Bank at Columbia University and the NIH Neuro-Biobank. The control hippocampal autopsy samples exhibited absence of neurological disorders and plaques, and previous experiments using these specific control samples had shown a lack of remodeling and leak in RyR2 (ref. 31). Information on the individuals with HF and controls is listed in Supplementary Tables 1 and 2. We obtained an Institutional Review Board exemption for the use of these specimens (AAAU3119).
Animal models
Six-month-old male C57BL/6 mice (Jackson laboratory), RyR2-p.Ser2808Ala and RyR2-p.Ser2808Asp mice (C57BL/6 background, available in the Marks Laboratory) were maintained and studied according to protocols approved by the Institutional Animal Care and Use Committee of Columbia University (reference no. AC-AAAC5453). Animals were randomly assigned to one of the designed groups. All in vivo animal experiments were performed by investigators blinded to genotype and treatment groups. To induce HF in C57BL/6 and p.Ser2808Ala mice, the proximal left anterior descendent coronary artery was ligated under general anesthesia using isoflurane (1.5%) in orally intubated mice as previously described84. The Rycal S107 (BBB permeant) was administered in drinking water at 75 mg per kg body weight per day for 1 month as previously described31. To differentiate between CNS effects versus peripheral cardiac effects, the Rycal ARM036 (non-BBB permeant)27 was administered in drinking water at 20 mg per kg body weight per day for 1 month. Propranolol (10 mg per kg body weight per day) and SD-208 (10 mg per kg body weight per day) treatments were given by intraperitoneal injection. Standard food was provided ad libitum throughout the experiments.
Echocardiography
Cardiac function was assessed in anesthetized mice (1–3% isoflurane, 100% oxygen) by transthoracic echocardiography using a high-resolution ultrasound system (Vevo 2100; VisualSonics) equipped with a 40-MHz linear array transducer (MS550, Vevo2100, VisualSonics). A left ventricular parasternal long-axis two-dimensional view in M-mode was performed at the level of papillary muscle to assess left ventricular wall thicknesses and internal diameters, allowing the calculation of the fractional shortening and ejection fraction by the Teicholz method as previously described53. HF animal models’ characteristics are shown in Supplementary Tables 3 and 6.
Behavioral studies
Open field
The behavior assays were performed 1 month after the initiation of the cardiac insult and last over several weeks. Briefly, the open field test was processed in a chamber with an area of 50 cm × 50 cm and walls of 38 cm in height31. Each mouse was placed at the center of the chamber and allowed to move freely for 6 min. The total time spent in the center area and peripheral area was recorded.
Elevated plus maze
The levels of disinhibited behavior were evaluated using the EPM test31,47. The maze was 40 cm high and contained two 61 cm × 5 cm open arms and two 61 cm × 5 cm × 15 cm closed arms with a center area of 5 cm × 5 cm. Briefly, each mouse was placed in the center area of the apparatus and allowed to move freely for 5 min. The total time spent in the open arm and close arm was recorded.
Novel object recognition
The short-term recognition memory was evaluated using the novel object recognition test as previously described31,48,85. The same open field arena was used for this test. One day after the open field test, each mouse was returned to the open field arena that contained two identical objects and allowed to freely explore for 10 min. After a 1-h interval, each mouse was placed back into the same arena, where one of the objects was replaced with a novel object, for another 5 min. The discrimination index of each mouse to explore the novel object was recorded and calculated.
Morris water maze
The MWM was used to study spatial learning and memory in MI mice and SHAM mice as controls31,49. The experimental apparatus included a water tank that had a diameter of 122 cm and a height of 82 cm, filled with water at 23 ± 2 °C and mixed with nontoxic white paint to make it opaque. The surface of the water pool was vertically divided into four quadrants. A plastic platform with a surface area of 10 cm × 10 cm and height of 55 cm was located in the middle of the northwest quadrant and 1 cm underneath the surface during the experiment. The experiment lasted for 5 d, including 4 d of training trials and 1 d of probe trial on day 5. During daily training trials, each mouse was released from different quadrants alternately in each trial. There were three trials per day and each trial lasted for 60 s. The duration for each mouse to find and sit on the platform for at least 2 s was defined as latency. On day 5 of the experiment, the hidden platform was removed, and each mouse was allowed to free swim for 60 s. The total duration spent in the quadrant with the previously hidden target (target quadrant) was recoded and the number of crossings through that quadrant (target crossing) was also recorded.
All the experiments were recorded by a camera installed over the experimental apparatus and analyzed by Etho Vision XT video tracking software (Noldue Information Technology). Two-way ANOVA analysis was performed for the training trials of MWM, and t-test and one-way ANOVA analysis were performed with Tukey’s test post hoc correction for the rest of the tests. Minimum statistically significant differences were established at P < 0.05.
Brain PET/CT imaging
For the brain glucose metabolism assessment, a 10-min static PET scan of brains was performed. After completing the behavioral assays, mice from different experimental groups were injected intravenously with 5.4–8.1 MBq (146–220 μCi) of [18F]FDG. Around 2 h after injection, brains were dissected and static 10-min PET images were obtained using an Inveon MicroPET scanner (Siemens).
For the brain blood flow assessment, a 2-min dynamic PET scan of mice was performed. MI and SHAM mice were injected intravenously with 4.0–4.3 MBq (109–117 mCi) of FDG, and 2-min dynamic PET scans were obtained using an Inveon microPET scanner (Siemens)86,87,88. The body temperature of mice was maintained at 37 °C. MicroCT (MILabs) was used for anatomical references. Regions of interest were manually drawn over the hippocampus. All PET images were reconstructed using an 3D-OSEM algorithm with three iterations in a 256 × 256 matrix (Inveon, Siemens) and analyzed using VivoQuant version 4 (Invicro). Decay correction was applied to all PET data.
Field excitatory postsynaptic potentials evaluation
LTP experiments were performed as previously described89. Briefly, transverse hippocampal slices (400 μm) were cut by tissue chopper and transferred to a recording chamber in which the temperature was maintained at 29 °C. During the recovery and recording periods, the slices were constantly perfused with artificial CSF (124.0 mM NaCl, 4.4 mM KCl, 1.0 mM Na2HPO4, 25.0 mM NaHCO3, 2.0 mM CaCl2, 2.0 mM MgCl2 and 10.0 mM glucose) that was bubbled with 95% O2 and 5% CO2. fEPSPs were evaluated at the Schaffer collateral by a bipolar electrode placed at the CA3 and recording at the CA1 with an artificial CSF-filled glass pipette. Basal synaptic transmission was evaluated via measurement of the I–O curve via plotting the relationship between increased voltages (5 V to 40 V) and evoked fEPSP responses. A 30-min baseline was recorded every minute at a voltage eliciting 35% of the maximum evoked response as determined by the I–O curve. LTP was elicited by a theta-burst stimulation (four pulses at 100 Hz, with the bursts repeated at 5 Hz and three tetani of ten-burst trains administered at 15-s intervals), and responses were recorded for 2 h. Results were analyzed in pClamp (Molecular Devices) and fEPSP slopes for I–O and LTP traces were compared by two-way ANOVA for repeated measures.
Hippocampal neurons culture and Ca2+ imaging
Hippocampi from WT and RyR2-p.Ser2808Asp pups were dissociated and cultured using Pierce primary neuron isolation kit (88280) according to manufacturer instructions. Briefly, hippocampi were dissected from one postnatal day mice and digested with 0.25% papain at 37 °C for 30 min. Digested cells were resuspended in pre-warmed serum-supplemented neuronal culture medium and plated in collagen/poly-d-lysine-coated 35-mm dishes with coverslip at 37 °C in a 5% CO2 incubator. After 24 h, the medium was replaced with an equivalent volume of serum-free neuronal culture medium. Next, 1× neuronal growth supplement was added at day 3 to reduce the non-neuronal cell contamination and maintain neurons at high purity during the culture period. Treated cells were incubated overnight with the following drugs: S107 (10 μM), isoproterenol (1 μM) and propranolol (1 μM). Cells were loaded with 10 μM fluo-4, AM, in culture medium for 30 min at 37 °C and then washed and incubated with Krebs solution (140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 11 mM glucose and 5 mM HEPES, pH 7.4). Imaging experiments were performed at room temperature (RT; 26 °C). Caffeine was prepared in Krebs solution and added to the cells at 10 mM. Time-series confocal imaging was performed by excitation with a 488-nm light from the argon laser of a Zeiss LSM 800 inverted confocal microscope (×40 oil immersion lens). Data were analyzed using ImageJ software.
Lysate preparation and western blots
Tissues (50 mg) were lysed using a Dounce homogenizer in 0.25 ml of 10 mM Tris maleate (pH 7.0) buffer with protease inhibitors (complete inhibitors from Roche). Samples were centrifuged at 800g for 20 min and the protein concentrations of the supernatants were determined by Bradford assay. To analyze protein expression in tissues, tissue lysates (20 μg) were separated by 4–20% SDS–PAGE and immunoblots were developed using antibodies against the indicated proteins (see list of antibodies used in Supplementary Information). Each protein was detected on a separate immunoblot.
Analyses of ryanodine receptor complex
RyR2 was immunoprecipitated from 0.1 mg of hippocampus lysate using an anti-RyR2-specific antibody (2 μg) in 0.5 ml of a modified radioimmune precipitation assay buffer (50 mm Tris-HCl, pH 7.2, 0.9% NaCl, 5.0 mm NaF, 1.0 mm Na3VO4, 1% Triton X-100 and protease inhibitors; RIPA) overnight at 4 °C. The immune complexes were incubated with protein A-Sepharose beads (Sigma) at 4 °C for 1 h, and the beads were washed three times with RIPA as previously described58. Then, 10 μl of immunoprecipitated volume was loaded on 6% gels to probe total phosphorylated, nitrosylated and total RyR2 or on 4–20% gradient gels to probe calstabin2 and NOX2. Proteins were transferred onto nitrocellulose membranes for 1 h at 200 mA. Immunoblots were developed using the following primary antibodies (see list of antibodies used in Supplementary Information): anti-RyR2, anti-phospho-RyR-Ser(P)-2808, anti-Cys-NO, anti-calstabin and anti-NOX2. The channel’s oxidation was determined by probing the carbonyl groups in the protein side chains by reaction with 2,4-dinitrophenylhydrazine. The DNP signal associated with RyR2 was determined using a specific anti-DNP antibody according to the manufacturer using an Odyssey system (LI-COR Biosciences) with infrared-labeled anti-mouse and anti-rabbit immunoglobulin G (IgG; 1:5,000 dilution) secondary antibodies. RyR2 PTM levels, calstabin2 and NOX2 binding were quantified using image studio (LI-COR Biosciences) and normalized to total immunoprecipitated RyR2 protein.
Protein kinase A activity assay
PKA activity in hippocampal lysates was determined using a PKA activity kit (Thermo Fisher, EIAPKA). Briefly, samples were added to a microtiter plate containing an immobilized PKA substrate that is phosphorylated by PKA in the presence of ATP. After incubating the samples with ATP at RT for 2 h, the plate was incubated with the phospho-PKA substrate antibody for 60 min. After washing the plate with wash buffer, goat anti-rabbit IgG horseradish peroxidase conjugate was added to each well. The plate was aspirated, washed, and TMB substrate was added to each well, which was then incubated for 30 min at RT. A plate reader was used to determine the absorbance at 450 nm. The sample signals were compared to a standard curve.
Calmodulin-dependent protein kinase II activity assay
CaMKII activity in hippocampal lysates was determined using the CycLex CaM kinase II Assay Kit (MBL International). Briefly, samples were added to a microtiter plate containing an immobilized CaMKII substrate that is phosphorylated by CaMKII in the presence of Mg2+ and ATP. After incubating the samples in kinase buffer containing Mg2+ and ATP at RT for 1 h, the plate was washed and incubated with the horseradish peroxidase-conjugated anti-phospho-CaMKII substrate antibody for 60 min. The plate was aspirated, washed, and TMB substrate was added to each well, which was then incubated for 30 min at RT. A plate reader was used to determine the absorbance at 450 nm. The sample signals were compared to a standard curve.
Endoplasmic reticulum vesicle preparation
Hippocampi were homogenized on ice in 300 mM sucrose, 20 mM PIPES (pH 7.0) in the presence of protease inhibitors (Roche), and centrifuged at 5,900g for 20 min at 4 °C. The supernatant was ultracentrifuged at 100,000g for 1 h at 4 °C. The final pellet containing microsomal fractions enriched in ER vesicles was resuspended and aliquoted in 300 mM sucrose, 5 mM PIPES (pH 7.0) containing protease inhibitors. Samples were frozen in liquid nitrogen and stored at −80 °C.
Single-channel data using planar lipid bilayers
Planar lipid bilayers were formed using a 3:1 mixture of phosphatidylethanolamine and phosphatidylcholine (Avanti Polar Lipids) suspended (30 mg ml−1) in decane by painting the lipid/decane solution across a 200-µm aperture in a polysulfonate cup (Warner Instruments) separating two chambers. The trans chamber (1 ml), representing the intra-ER/SR (luminal) compartment, was connected to the headstage input of a bilayer voltage clamp amplifier (BC-525D, Warner Instruments) and the cis chamber (1 mL), representing the cytoplasmic compartment, was held at virtual ground. Solutions in both chambers were as follows: 1 mM EGTA, 250/125 mM HEPES/Tris, 50 mM KCl, 0.64 mM CaCl2, pH 7.35 as cis solution and 53 mM Ca(OH)2, 50 mM KCL, 250 mM HEPES, pH 7.35 as trans solution. The concentration of free Ca2+ in the cis chamber was calculated using the WinMaxC program (version 2.50; https://somapp.ucdmc.ucdavis.edu/pharmacology/bers/maxchelator/webmaxc/webmaxcE.htm). ER vesicles were added to the cis side, and fusion with the lipid bilayer was induced by making the cis side hyperosmotic by the addition of 400–500 mM KCl. After the appearance of potassium and chloride channels, the cis compartment was perfused with the cis solution. Single-channel currents were recorded at 0 mV by using a Bilayer Clamp BC-535 amplifier (Warner Instruments), filtered at 1 kHz, and digitized at 4 kHz. All experiments were performed at RT. Data acquisitions were performed using Digidata 1440A and Axoscope 10.2 software, and recordings were analyzed using Clampfit 10.2 (Molecular Devices). Open probability was identified by 50% threshold analyses using a minimum of 2 min of continuous recording. At the conclusion of each experiment, ryanodine (5 µM) was added to the cis chamber to confirm channels as RyR as previously described51.
Endoplasmic reticulum Ca2+ leak assay
ER microsomes (5 μg ml−1) were diluted into a buffer (pH 7.2) containing 8 mM k-phosphocreatine, and 2 units per ml of creatine kinase, mixed with 3 μM Fluo-4 and added to multiple wells of a 96-well plate. Ca2+ loading of the microsomes was initiated by adding 1 mM ATP. After Ca2+ uptake (50 s), 3 μM thapsigargin was added to inhibit the Ca2+ reuptake by sarco-endoplasmic reticulum Ca2+-ATPase. ER Ca2+ leak was measured by the increase in intensity of the Fluo-4 signal (measured in a Tecan fluorescence plate reader). The Ca2+ leak was quantified as the percentage of uptake.
Norepinephrine, cardiac troponin I, brain natriuretic peptide and blood gas levels
Hippocampal norepinephrine, cardiac troponin I and brain natriuretic peptide plasma levels were determined by using commercially available ELISA kits (Biomatik and Elabscience, respectively) according to the manufacturer’s instructions. Blood gas analyses was performed using CG8+ cartridges and the i-STAT1 analyzer from ABBOTT, according to the manufacturer’s instructions.
Isolation of mitochondria
Mice hippocampi were removed, washed with PBS, and the mitochondria were isolated as previously described90. Briefly, tissues were placed in a homogenization medium at 5 ml g–1 of tissue (320 mM sucrose, 225 mM mannitol, 5 mM Tris-Hcl, pH 7.4). Tissues were homogenized in a glass Potter and centrifuged for 3 min at 1.330g at 4 °C. The pellet was then discarded, and the supernatant was centrifuged for 10 min at 21,000g. The mitochondrial pellet was resuspended in 15 ml of the mitochondrial isolation buffer (75 mM sucrose, 225 mM mannitol, 5 mM Tris-Hcl, pH 7.4) and centrifuged again for 10 min at 21,000g at 4 °C. Pellet was resuspended in the mitochondrial isolation assay and assessed for protein concentration using a Bradford assay.
Mitochondrial Ca2+ content
In total, 20 μg of isolated mitochondria was sonicated and used to measure the mitochondrial Ca2+ content using the o-cresolphthalein complexone assay (Cayman Chemical) according to the manufacturer’s instructions and as previously described46.
Mitochondrial reactive oxygen species production
Isolated mitochondria (20 μg) were incubated in VO2 buffer (250 mM sucrose, 50 mM KCl, 25 Mm Tris·HCl, and 10 mM K2HPO4, pH 7.4) in a 96-well black plate. ROS production was assessed at 37 °C for 60 min during states 3 and 4 respiration by adding respiration substrates before the addition of 50 μM dichlorodihydrofluorescein diacetate (H2DCFDA, Invitrogen). ROS production is directly proportional to fluorescence emission monitored at an excitation of 485 nm and emission of 528 nm with a microplate fluorimeter. Microplate data were compiled and analyzed using i-control Microplate Reader Software and results were expressed as arbitrary fluorescence units91.
Global quantitative proteomics analysis
For global quantitative proteomics of fresh frozen hippocampal samples from MI and SHAM mice, diaPASEF92 (data-independent acquisition)-based proteomics were used. The cutoff values for differentially expressed proteins included P value < 0.05 (permutation-based FDR correction), fold change ≥ 1.5 and unique peptides ≥ 2 (volcano plot). The significantly changed proteins between MI and SHAM hippocampus were used for heat map, GO and KEGG analysis. Gene-set enrichment analyses also was performed to identify the statistically significant gene sets in a ranked gene list. More details are described in the Supplementary Information.
Statistics
Data are presented as individual values with the mean ± s.e.m. Normal distribution was tested by Shapiro–Wilk normality and log normality tests. Statistical analyses were performed using an unpaired two-tailed Student’s t-test, and one- or two-way ANOVA with Tukey’s test post hoc correction for multiple comparisons. Minimum statistically significant differences were established at P < 0.05. No statistical methods were used to predetermine sample sizes, but our sample sizes are similar to those reported in previous publications26. Animals who died during the behavioral tests were excluded. No data were excluded for the remaining experiments.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data supporting the findings of this study are documented within the paper and Supplementary Information. Proteomics data are deposited at PRIDE under accession number PXD042295. No custom software codes were used. RNA-sequencing data are deposited on the Sequence Read Archive under accession number PRJNA956662. Data are accessible at the Center for Computational Mass Spectrometry MassIVE resource under accession MSV000091695. Source data are provided with this paper.
References
Ambrosy, A. P. et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J. Am. Coll. Cardiol. 63, 1123–1133 (2014).
Ponikowski, P. et al. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 1, 4–25 (2014).
Leto, L. & Feola, M. Cognitive impairment in heart failure patients. J. Geriatr. Cardiol. 11, 316–328 (2014).
McParland, C., Krishnan, B., Wang, Y. & Gallagher, C. G. Inspiratory muscle weakness and dyspnea in chronic heart failure. Am. Rev. Respir. Dis. 146, 467–472 (1992).
Huynh, K. Heart failure: HF-induced diaphragmatic atrophy and weakness. Nat. Rev. Cardiol. 14, 384 (2017).
Vogels, R. L. et al. Profile of cognitive impairment in chronic heart failure. J. Am. Geriatr. Soc. 55, 1764–1770 (2007).
Levin, S. N. et al. Cognitive status in patients hospitalized with acute decompensated heart failure. Am. Heart J. 168, 917–923 (2014).
Vogels, R. L., Scheltens, P., Schroeder-Tanka, J. M. & Weinstein, H. C. Cognitive impairment in heart failure: a systematic review of the literature. Eur. J. Heart Fail. 9, 440–449 (2007).
Dickson, V. V., Tkacs, N. & Riegel, B. Cognitive influences on self-care decision making in persons with heart failure. Am. Heart J. 154, 424–431 (2007).
Wu, J. R. et al. Factors influencing medication adherence in patients with heart failure. Heart Lung 37, 8–16 (2008).
Bouvy, M. L. et al. Effect of a pharmacist-led intervention on diuretic compliance in heart failure patients: a randomized controlled study. J. Card. Fail. 9, 404–411 (2003).
Evangelista, L. S., Berg, J. & Dracup, K. Relationship between psychosocial variables and compliance in patients with heart failure. Heart Lung 30, 294–301 (2001).
Murad, K. et al. Burden of comorbidities and functional and cognitive impairments in elderly patients at the initial diagnosis of heart failure and their impact on total mortality: the cardiovascular health study. JACC Heart Fail. 3, 542–550 (2015).
Hammond, C. A. et al. Long-term cognitive decline after newly diagnosed heart failure: longitudinal analysis in the CHS (Cardiovascular Health Study). Circ. Heart Fail. 11, e004476 (2018).
van den Hurk, K. et al. Heart failure and cognitive function in the general population: the Hoorn Study. Eur. J. Heart Fail. 13, 1362–1369 (2011).
Alosco, M. L. et al. Cerebral perfusion is associated with white matter hyperintensities in older adults with heart failure. Congest. Heart Fail. 19, E29–E34 (2013).
Jefferson, A. L. et al. Lower cardiac output is associated with greater white matter hyperintensities in older adults with cardiovascular disease. J. Am. Geriatr. Soc. 55, 1044–1048 (2007).
Hartupee, J. & Mann, D. L. Neurohormonal activation in heart failure with reduced ejection fraction. Nat. Rev. Cardiol. 14, 30–38 (2017).
Daniele, G., DiLucia, S., Masci, P. G. & Del Monte, F. Heart and brain: complex relationships for left ventricular dysfunction. Curr. Cardiol. Rep. 22, 72 (2020).
Sara, S. J. & Bouret, S. Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron 76, 130–141 (2012).
Reimer, J. et al. Pupil fluctuations track fast switching of cortical states during quiet wakefulness. Neuron 84, 355–362 (2014).
van den Brink, R. L. et al. Catecholaminergic neuromodulation shapes intrinsic MRI functional connectivity in the human brain. J. Neurosci. 36, 7865–7876 (2016).
Eschenko, O., Magri, C., Panzeri, S. & Sara, S. J. Noradrenergic neurons of the locus coeruleus are phase locked to cortical up-down states during sleep. Cereb. Cortex 22, 426–435 (2012).
McGinley, M. J., David, S. V. & McCormick, D. A. Cortical membrane potential signature of optimal states for sensory signal detection. Neuron 87, 179–192 (2015).
Francis, G. S. et al. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the studies of left ventricular dysfunction (SOLVD). Circulation 82, 1724–1729 (1990).
Lacampagne, A. et al. Post-translational remodeling of ryanodine receptor induces calcium leak leading to Alzheimer’s disease-like pathologies and cognitive deficits. Acta Neuropathol. 134, 749–767 (2017).
Dridi, H. et al. Role of defective calcium regulation in cardiorespiratory dysfunction in Huntington’s disease. JCI Insight https://doi.org/10.1172/jci.insight.140614 (2020).
Surmeier, D. J. et al. Calcium and Parkinson’s disease. Biochem. Biophys. Res. Commun. 483, 1013–1019 (2017).
Augustine, G. J., Santamaria, F. & Tanaka, K. Local calcium signaling in neurons. Neuron 40, 331–346 (2003).
Hoffman, D. A., Sprengel, R. & Sakmann, B. Molecular dissection of hippocampal theta-burst pairing potentiation. Proc. Natl Acad. Sci. USA 99, 7740–7745 (2002).
Liu, X. et al. Role of leaky neuronal ryanodine receptors in stress-induced cognitive dysfunction. Cell 150, 1055–1067 (2012).
Santulli, G. & Marks, A. R. Essential roles of intracellular calcium release channels in muscle, brain, metabolism and aging. Curr. Mol. Pharmacol. 8, 206–222 (2015).
Mateos-Aparicio, P. & Rodriguez-Moreno, A. Calcium dynamics and synaptic plasticity. Adv. Exp. Med. Biol. 1131, 965–984 (2020).
Zalk, R. et al. Structure of a mammalian ryanodine receptor. Nature 517, 44–49 (2015).
Marx, S. O. et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101, 365–376 (2000).
Lehnart, S. E. et al. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell 123, 25–35 (2005).
Meissner, G. & Henderson, J. S. Rapid calcium release from cardiac sarcoplasmic reticulum vesicles is dependent on Ca2+ and is modulated by Mg2+, adenine nucleotide, and calmodulin. J. Biol. Chem. 262, 3065–3073 (1987).
Kushnir, A., Shan, J., Betzenhauser, M. J., Reiken, S. & Marks, A. R. Role of CaMKIIdelta phosphorylation of the cardiac ryanodine receptor in the force frequency relationship and heart failure. Proc. Natl Acad. Sci. USA 107, 10274–10279 (2010).
Shan, J. et al. Phosphorylation of the ryanodine receptor mediates the cardiac fight or flight response in mice. J. Clin. Invest. 120, 4388–4398 (2010).
Reiken, S. et al. PKA phosphorylation activates the calcium release channel (ryanodine receptor) in skeletal muscle: defective regulation in heart failure. J. Cell Biol. 160, 919–928 (2003).
Marks, A. R. A guide for the perplexed: towards an understanding of the molecular basis of heart failure. Circulation 107, 1456–1459 (2003).
Rullman, E. et al. Modifications of skeletal muscle ryanodine receptor type 1 and exercise intolerance in heart failure. J. Heart Lung Transplant. 32, 925–929 (2013).
Lunde, P. K. et al. Effects of congestive heart failure on Ca2+ handling in skeletal muscle during fatigue. Circ. Res. 98, 1514–1519 (2006).
Santulli, G. et al. Calcium release channel RyR2 regulates insulin release and glucose homeostasis. J. Clin. Invest. 125, 4316 (2015).
Marx, S. O. et al. Phosphorylation-dependent regulation of ryanodine receptors: a novel role for leucine/isoleucine zippers. J. Cell Biol. 153, 699–708 (2001).
Santulli, G., **e, W., Reiken, S. R. & Marks, A. R. Mitochondrial calcium overload is a key determinant in heart failure. Proc. Natl Acad. Sci. USA 112, 11389–11394 (2015).
Komada, M., Takao, K. & Miyakawa, T. Elevated plus maze for mice. J. Vis. Exp. https://doi.org/10.3791/1088 (2008).
Bevins, R. A. & Besheer, J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat. Protoc. 1, 1306–1311 (2006).
Morris, M. D. & Gebhart, G. F. Antianxiety agents and emotional behavior, an information processing analysis. Prog. Neuropsychopharmacol. 5, 219–240 (1981).
Miotto, M. C. et al. Structural analyses of human ryanodine receptor type 2 channels reveal the mechanisms for sudden cardiac death and treatment. Sci. Adv. 8, eabo1272 (2022).
Melville, Z. et al. A drug and ATP binding site in type 1 ryanodine receptor. Structure 30, 1025–1034 (2022).
Rocher, A. B., Chapon, F., Blaizot, X., Baron, J. C. & Chavoix, C. Resting-state brain glucose utilization as measured by PET is directly related to regional synaptophysin levels: a study in baboons. Neuroimage 20, 1894–1898 (2003).
Shan, J. et al. Role of chronic ryanodine receptor phosphorylation in heart failure and beta-adrenergic receptor blockade in mice. J. Clin. Invest. 120, 4375–4387 (2010).
Bussiere, R. et al. Amyloid beta production is regulated by beta2-adrenergic signaling-mediated post-translational modifications of the ryanodine receptor. J. Biol. Chem. 292, 10153–10168 (2017).
Rothman, J. E. The principle of membrane fusion in the cell (Nobel lecture). Angew. Chem. Int. Ed. Engl. 53, 12676–12694 (2014).
Webster, K. M. et al. Inflammation in epileptogenesis after traumatic brain injury. J. Neuroinflammation 14, 10 (2017).
Das, P. & Golde, T. Dysfunction of TGF-beta signaling in Alzheimer’s disease. J. Clin. Invest. 116, 2855–2857 (2006).
Dridi, H. et al. Ryanodine receptor remodeling in cardiomyopathy and muscular dystrophy caused by lamin A/C gene mutation. Hum. Mol. Genet. 29, 3919–3934 (2021).
Souvannakitti, D. et al. NADPH oxidase-dependent regulation of T-type Ca2+ channels and ryanodine receptors mediate the augmented exocytosis of catecholamines from intermittent hypoxia-treated neonatal rat chromaffin cells. J. Neurosci. 30, 10763–10772 (2010).
Donoso, P. et al. Stimulation of NOX2 in isolated hearts reversibly sensitizes RyR2 channels to activation by cytoplasmic calcium. J. Mol. Cell. Cardiol. 68, 38–46 (2014).
Grossmann, D., Berenguer-Escuder, C., Chemla, A., Arena, G. & Kruger, R. The Emerging role of RHOT1/Miro1 in the pathogenesis of Parkinson’s disease. Front. Neurol. 11, 587 (2020).
Saito, K., Elce, J. S., Hamos, J. E. & Nixon, R. A. Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer disease: a potential molecular basis for neuronal degeneration. Proc. Natl Acad. Sci. USA 90, 2628–2632 (1993).
Reiken, S., Sittenfeld, L., Dridi, H., Liu, Y., Liu, X. & Marks, A. R. Alzheimer’s-like signaling in brains of COVID-19 patients. Alzheimers. Dement. 18, 955–965 (2022).
Grassi, G., Quarti-Trevano, F. & Esler, M. D. Sympathetic activation in congestive heart failure: an updated overview. Heart Fail. Rev. https://doi.org/10.1007/s10741-019-09901-2 (2019).
Rundqvist, B., Elam, M., Bergmann-Sverrisdottir, Y., Eisenhofer, G. & Friberg, P. Increased cardiac adrenergic drive precedes generalized sympathetic activation in human heart failure. Circulation 95, 169–175 (1997).
Suzuki, Y. J. & Ford, G. D. Redox regulation of signal transduction in cardiac and smooth muscle. J. Mol. Cell. Cardiol. 31, 345–353 (1999).
Zima, A. V. & Blatter, L. A. Redox regulation of cardiac calcium channels and transporters. Cardiovasc. Res. 71, 310–321 (2006).
Waning, D. L. et al. Excess TGF-beta mediates muscle weakness associated with bone metastases in mice. Nat. Med. 21, 1262–1271 (2015).
Manaenko, A., Lekic, T., Barnhart, M., Hartman, R. & Zhang, J. H. Inhibition of transforming growth factor-beta attenuates brain injury and neurological deficits in a rat model of germinal matrix hemorrhage. Stroke 45, 828–834 (2014).
Chompre, G., Martinez-Orengo, N., Cruz, M., Porter, J. T. & Noel, R. J. Jr. TGFbetaRI antagonist inhibits HIV-1 Nef-induced CC chemokine family ligand 2 (CCL2) in the brain and prevents spatial learning impairment. J. Neuroinflammation 16, 262 (2019).
de Streel, G. & Lucas, S. Targeting immunosuppression by TGF-beta1 for cancer immunotherapy. Biochem. Pharmacol. 192, 114697 (2021).
Gelber, R. P. et al. Antihypertensive medication use and risk of cognitive impairment: the Honolulu-Asia Aging Study. Neurology 81, 888–895 (2013).
Hajjar, I. et al. Cross-sectional and longitudinal association between antihypertensive medications and cognitive impairment in an elderly population. J. Gerontol. A Biol. Sci. Med. Sci. 60, 67–73 (2005).
Hahn, B., Olmstead, C. K., Yuille, M. B., Chiappelli, J. J. & Wells, A. K. Attention-enhancing effects of propranolol and synergistic effects with nicotine. Cogn. Affect. Behav. Neurosci. 20, 658–668 (2020).
Dobarro, M., Gerenu, G. & Ramirez, M. J. Propranolol reduces cognitive deficits, amyloid and tau pathology in Alzheimer’s transgenic mice. Int. J. Neuropsychopharmacol. 16, 2245–2257 (2013).
Holm, H. et al. Beta-blocker therapy and risk of vascular dementia: a population-based prospective study. Vascul. Pharmacol. 125-126, 106649 (2020).
Steinman, M. A. et al. Association of beta-blockers with functional outcomes, death and rehospitalization in older nursing home residents after acute myocardial infarction. JAMA Intern. Med. 177, 254–262 (2017).
Huson, V. & Regehr, W. G. Diverse roles of synaptotagmin-7 in regulating vesicle fusion. Curr. Opin. Neurobiol. 63, 42–52 (2020).
Masliah, E. et al. Altered expression of synaptic proteins occurs early during progression of Alzheimer’s disease. Neurology 56, 127–129 (2001).
Sze, C. I., Bi, H., Kleinschmidt-DeMasters, B. K., Filley, C. M. & Martin, L. J. Selective regional loss of exocytotic presynaptic vesicle proteins in Alzheimer’s disease brains. J. Neurol. Sci. 175, 81–90 (2000).
Brinkmalm, A. et al. SNAP-25 is a promising novel cerebrospinal fluid biomarker for synapse degeneration in Alzheimer’s disease. Mol. Neurodegener. 9, 53 (2014).
Ohrfelt, A. et al. The pre-synaptic vesicle protein synaptotagmin is a novel biomarker for Alzheimer’s disease. Alzheimers Res. Ther. 8, 41 (2016).
Reiken, S. et al. Alzheimer’s-like signaling in brains of COVID-19 patients. Alzheimers Dement. https://doi.org/10.1002/alz.12558 (2022).
Wehrens, X. H. et al. Enhancing calstabin binding to ryanodine receptors improves cardiac and skeletal muscle function in heart failure. Proc. Natl Acad. Sci. USA 102, 9607–9612 (2005).
Leger, M. et al. Object recognition test in mice. Nat. Protoc. 8, 2531–2537 (2013).
Cochet, A. et al. Evaluation of breast tumor blood flow with dynamic first-pass 18F-FDG PET/CT: comparison with angiogenesis markers and prognostic factors. J. Nucl. Med. 53, 512–520 (2012).
Mullani, N. A. & Gould, K. L. First-pass measurements of regional blood flow with external detectors. J. Nucl. Med. 24, 577–581 (1983).
Mullani, N. A. et al. Tumor blood flow measured by PET dynamic imaging of first-pass 18F-FDG uptake: a comparison with 15O-labeled water-measured blood flow. J. Nucl. Med. 49, 517–523 (2008).
Puzzo, D. et al. Tau is not necessary for amyloid-beta-induced synaptic and memory impairments. J. Clin. Invest. 130, 4831–4844 (2020).
Suski, J. M. et al. Relation between mitochondrial membrane potential and ROS formation. Methods Mol. Biol. 810, 183–205 (2012).
Dridi, H. et al. Mitochondrial oxidative stress induces leaky ryanodine receptor during mechanical ventilation. Free Radic. Biol. Med. 146, 383–391 (2020).
Meier, F. et al. diaPASEF: parallel accumulation-serial fragmentation combined with data-independent acquisition. Nat. Methods 17, 1229–1236 (2020).
Acknowledgements
This work was supported by grants from the NIH to A.R.M. (T32HL120826, R01HL145473, R01DK118240, R01HL142903, R01HL140934, R01AR070194, R25HL156002). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. We thank J. Mann, A. Molotkov and A. Mintz (NYSPI) for their guidance and recommendations in performing the brain imaging experiments. We thank M. Yang for help in the interpretation of the behavioral assays. We thank H. Li for her guidance in the mouse hippocampi dissection and culture.
Author information
Authors and Affiliations
Contributions
H.D. and A.R.M. designed experiments, analyzed data and edited/wrote the paper. Y.L., S.R., X.L., E.K.A., Q.Y., M.C.M., L.S., A.M., R.K.S., O.A. and A.L. designed experiments, analyzed data and edited the paper.
Corresponding authors
Ethics declarations
Competing interests
Columbia University and A.R.M. own stock in ARMGO, a company develo** compounds targeting RyR and have patents on Rycals. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Mark Mattson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Mouse model of leaky RyR2 (constitutive RyR2 PKA-phosphorylation) is associated with cognitive dysfunction.
Mouse model of leaky RyR2 (phospho-mimetic mutation) is associated with cognitive dysfunction. a) Open field test of SHAM (n = 14), S2808A-SHAM (n = 8), S2808A-MI (n = 8), S2808D (n = 13), and S2808D + S107 (n = 8) mice. Ratios of total time spent in the center area versus periphery area within first (1st) 3 min and second (2nd) 3 min are shown. b) Elevated plus maze test in SHAM (n = 14), S2808A-SHAM (n = 8), S2808A-MI (n = 8), S2808D (n = 13), and S2808D + S107 (n = 8) mice. Ratios of time spent on the open-arm versus closed-arm are shown. c) Novel object recognition test in SHAM (n = 14), S2808A-SHAM (n = 8), S2808A-MI (n = 8), S2808D (n = 13), and S2808D + S107 (n = 8) mice. Discrimination index is shown. d) Morris water maze test (learning curves) in SHAM (n = 14), S2808A-SHAM (n = 8), S2808A-MI (n = 8), S2808D (n = 13), and S2808D + S107 (n = 8) mice. e) Probe trials after escape platform removed in the same groups showing the total duration spent in the target quadrant. f) Number of target crossings SHAM (n = 14), S2808A-SHAM (n = 8), S2808A-MI (n = 8), S2808D (n = 13), and S2808D + S107 (n = 8) mice. g) Heat maps showing the latency from each group at Day 2 and Day 4. Individual values are shown with mean ± SEM (t-test * p < 0.05 in panel A shows significance between the first 3 min and second 3 min of the same groups. One-way ANOVA was used to compare the difference between the 5 groups in panel B, C, E and F; Two-way ANNOVA was used in panel D. Tukey’s test post-hoc correction for multiple comparisons was used; * p < 0.05, S2808A-SHAM vs. S2808D or S2808D + S107; # p < 0.05, S2808D vs. S2808D + S107. No differences were detected between S2808A-SHAM and S2808A-MI. All statistical tests were two-sided. Data are derived from biologically independent samples.
Extended Data Fig. 2 Cognitive function in RyR1-S2844D mice.
a) Open field test using WT mice (n = 10) and a mouse model with leaky RyR1 channels (S2844D) (n = 21). Ratios of total time spent in the center area versus periphery area within first 3 min and second 3 min are shown. b) Elevated plus maze test in WT mice (n = 10) and S2808D (n = 21). Ratios of time spent in the open-arm versus closed-arm are shown. c) Novel object recognition test in WT mice (n = 10) and S2808D (n = 21). Discrimination index is shown. d) Morris water maze test (learning curves) in WT mice (n = 10) and S2808D (n = 21). e) Probe trials after escape platform removed in the same groups showing the total duration spent in the target quadrant in WT mice (n = 10) and S2808D (n = 21). f) Number of target crossings in WT mice (n = 10) and S2808D (n = 21). g) Heat maps showing the latency from each group at Day 2 and Day 5. Individual values are shown with mean ± SEM. T-test was used in panel A-C, E-F, * p < 0.05 in panel A shows significance between the first 3 min and second 3 min of each group). Two-way ANOVA was used in panel D. Tukey’s test post-hoc correction for multiple comparisons was used. All statistical tests were two-sided. Data are derived from biologically independent samples.
Extended Data Fig. 3 Constitutive RyR2 phosphorylation on Ser2808 (S2808D mice) induces ER Ca2+ leak in the hippocampus.
Phospho-mimetic mutation (RyR2-S2808D mice) induces ER Ca2+ leak in the hippocampus. a, b) Representative SDS-PAGE analysis and quantification of modified RyR2 and calstabin2 immunoprecipitated from hippocampus of S2808A-SHAM (n = 4), S2808A-MI (n = 4), S2808D (n = 4), S2808D + S107 mice (n = 4) (IP RyR2: Bands normalized to total RyR2); n = 4 in each group. c) ER Ca2+ leak measured in microsomes from hippocampi of S2808A-SHAM (n = 4), S2808A-MI (n = 4), S2808D, S2808D + S107 mice (n = 4). d) Bar graphs represent the quantification of Ca2+ leak as the percentage of uptake in all the experimental groups (n = 4 per group). e) Single-channel traces of RyR2 incorporated in planar lipid bilayers with 150 nM Ca2+ in the cis chamber, corresponding to representative experiments performed with hippocampal samples from S2808A-SHAM, S2808A-MI, S2808D, S2808D + S107 mice. f–h) RyR2 open probability (Po), mean open time (To), and mean close time (Tc) in S2808A-SHAM, S2808A-MI, S2808D, and S2808D + S107 mice (n = n = 5, 5, 4 and 4 respectively). Individual values are shown with mean ± SEM. One way-ANOVA and Tukey’s test post-hoc correction for multiple comparisons shows * p < 0.05, S2808A-SHAM vs. S2808D or S2808D + S107; # p < 0.05, S2808D vs. S2808D + S107. No differences were detected between S2808A-SHAM and S2808A-MI. All statistical tests were two-sided. Data are derived from biologically independent samples.
Extended Data Fig. 4 TGF-β activation in HF.
a) Immunoblots showing expressing levels of TGF-β, phosphorylated SMAD3, total SMAD3, and NOX2 binding to RyR2 in the hippocampi of controls (n = 4) and HF patients (n = 9). b) Bar graphs depicting the ratio of TGF-β expression normalized to GAPDH, phosphorylated SMAD3 to total SMAD3 and NOX2 binding to RyR2 (IP RyR2). The same quantity of proteins were loaded on two separate gels and blotted separately for SMAD3 and pSMAD3. Individual values are shown with mean ± SEM (t-test * p < 0.05, Controls vs. HF patients). c) Immunoblots showing expressing levels of TGF-β, phosphorylated SMAD3, total SMAD3, and NOX2 binding to RyR2 in the hippocampi of SHAM, MI, MI + ARM036, MI + S107, MI+ propranolol and MI + SD-208 mice (n = 6, 6, 6, 6, 4 and 4 respectively). d) Bar graphs depicting the ratio of TGF-β expression normalized to GAPDH, phosphorylated SMAD3 to total SMAD3 and NOX2 binding to RyR2 (IP RyR2). The same quantity of proteins were loaded on two separate gels and blotted separately for SMAD3 and pSMAD3. Individual values are shown with mean ± SEM. One-way ANOVA and Tukey’s test post-hoc correction for multiple comparisons shows * p < 0.05, SHAM vs. MI, MI + ARM036 or MI + S107; #p < 0.05, MI vs. MI + S107, MI+ propranolol or MI + SD-208. All statistical tests were two-sided. Data are derived from biologically independent samples.
Extended Data Fig. 5 Pre-ranked gene set enrichment analysis (GSEA) of the hippocampal proteomics.
Dot plots show: a) Top 20 up- and top 20 down-regulated GO biological process, b) top 10 up- and top 20 down-regulated GO cellular component, c) top 10 up- and top 20 down-regulated GO molecular function terms. Significantly changed protein abundance was determined by unpaired t-test with a threshold for significance of p < 0.05 (permutation-based FDR correction), fold-change ≥1.5, unique peptides ≥2. Data are derived from biologically independent samples. All statistical tests were two-sided.Source file PRIDE #PXD042295.
Extended Data Fig. 6 Gene set enrichment analysis (GSEA) of the hippocampal proteomics.
The enrichment plots of representative KEGG pathway gene sets demonstrate that oxidative phosphorylation (a), Parkinson’s disease (b), Alzheimer’s disease (c), and Huntington’s disease (d) are significantly enriched in MI compared to SHAM. The heatmap on the right side of each panel visualizes the genes contributing to the enriched pathways. For the detailed list see Supplementary Table 8. Signal-to-noise ratio was used to rank the genes per their correlation with either MI phenotype (red) or SHAM phenotype (blue). The y-axis represents enrichment score (ES) and on the x-axis are genes (vertical black lines) represented in gene sets. The GSEA analysis calculates an enrichment score (the maximum deviation from zero) reflecting the degree of over-representation of a gene set at the top or the bottom of the ranked gene list. A positive ES indicates gene set enrichment at the top of the ranked list; a negative ES indicates gene set enrichment at the bottom of the ranked list. NES, normalized enrichment score; FDR, FDR adjusted p-value.
Extended Data Fig. 7 RNA sequencing analysis.
RNA-sequencing was performed on the hippocampi of SHAM and MI mice (n = 4 for each group). a) The Volcano plot shows differentially expressed genes (p-adj<0.05, fold-change ≥1.3) in SHAM and MI mice. Red indicates up-regulated, while blue represents down-regulated genes. Black indicates unchanged expression levels. b) The heat map shows significantly dysregulated genes (down-regulated: 2003, up-regulated: 1149 genes), the color scale bar shows the row normalized log2 protein abundance. c) Dot plots show top 10 GO biological processes, d) molecular functions, e) cellular components, and f) KEGG pathways that were enriched from differentially expressed genes. Significantly changed gene abundance was determined by unpaired t-test with a threshold for significance of p < 0.05 (permutation-based FDR correction), fold-change ≥1.5. Data are derived from biologically independent samples. All statistical tests were two-sided. See Supplementary Table 9for gene list. Data are accessible on SRA- Accession: PRJNA956662.
Extended Data Fig. 8 Pre-ranked gene set enrichment analysis (GSEA) of RNA sequencing.
Dot plots show: a) Top 20 up- and top 20 down-regulated GO biological process, b) top 20 up- and top 20 down-regulated GO cellular component, c) top 20 up- and top 20 down-regulated GO molecular function terms. Significantly changed gene abundance was determined by unpaired t-test with a threshold for significance of p < 0.05 (permutation-based FDR correction), fold-change ≥1.5. Data are derived from biologically independent samples. All statistical tests were two-sided.
Extended Data Fig. 9 Gene set enrichment analysis (GSEA) of the hippocampal RNA sequencing.
The enrichment plots of representative KEGG pathway gene sets demonstrate that oxidative phosphorylation (a), Parkinson’s disease (b), Alzheimer’s disease (c), and Huntington’s disease (d) are significantly enriched in MI compared to SHAM. The heatmap on the right side of each panel visualizes the genes contributing to the enriched pathways. For the detailed list, see Supplementary Table 10. Signal-to-Noise ratio was used to rank the genes per their correlation with either MI phenotype (red) or SHAM phenotype (blue). The y-axis represents enrichment score (ES) and on the x-axis are genes (vertical black lines) represented in gene sets. The GSEA analysis calculates an enrichment score (the maximum deviation from zero) reflecting the degree of over-representation of a gene set at the top or the bottom of the ranked gene list. A positive ES indicates gene set enrichment at the top of the ranked list; a negative ES indicates gene set enrichment at the bottom of the ranked list. NES, normalized enrichment score; FDR, FDR adjusted p-value.
Extended Data Fig. 10 Mitochondrial Ca2+ overload and oxidative stress in HF.
a) Cohort plot representation of differentially expressed mitochondrial proteins (SHAM vs MI) from 4 significantly enriched mitochondrial GO-terms and generated by GOplot. The color map represents fold change of proteins (log2 scale). b) Ca2+ accumulation in isolated mitochondria from SHAM (n = 6), MI (n = 5), MI + ARM036 (n = 5), and MI + S107 (n = 5) mice. C) Reactive oxygen species (ROS) production in isolated mitochondria from SHAM (n = 6), MI (n = 6), MI + ARM036 (n = 6), and MI + S107 (n = 5) mice. Individual values are shown with mean ± SEM (one-way ANOVA and Tukey’s test post-hoc correction for multiple comparisons show * p < 0.05, SHAM vs. MI or MI + ARM036; #p < 0.05, MI vs. MI + S107). All statistical tests were two-sided.
Supplementary information
Supplementary Information
Extended methods. List of used drugs. List of used antibodies. Supplementary Figs. 1 and 2 and Extended Figs. 1–10. Full uncut gels shown in the supplementary figures. Used RStudio codes. References.
Supplementary Tables 1–10
Supplementary information on the human specimens, the animal models and the sequencing data.
Source data
Source Data Fig. 1
Unprocessed western blots and statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Unprocessed western blots and statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Unprocessed western blots and statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dridi, H., Liu, Y., Reiken, S. et al. Heart failure-induced cognitive dysfunction is mediated by intracellular Ca2+ leak through ryanodine receptor type 2. Nat Neurosci 26, 1365–1378 (2023). https://doi.org/10.1038/s41593-023-01377-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-023-01377-6
- Springer Nature America, Inc.
This article is cited by
-
Expression of expanded GGC repeats within NOTCH2NLC causes cardiac dysfunction in mouse models
Cell & Bioscience (2023)