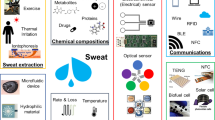
Abstract
The potential of monitoring biomarkers in sweat for health-related applications has spurred rapid growth in the field of wearable sweat sensors over the past decade. Some of the key challenges have been addressed, including measuring sweat-secretion rate and collecting sufficient sample volumes for real-time, continuous molecular analysis without intense exercise. However, except for assessment of cystic fibrosis and regional nerve function, the ability to accurately measure analytes of interest and their physiological relevance to health metrics remain to be determined. Although sweat is not a crystal ball into every aspect of human health, we expect sweat measurements to continue making inroads into niche applications involving active sweating, such as hydration monitoring for athletes and physical laborers and later for medical and casual health monitoring of relevant drugs and hormones.



Similar content being viewed by others
References
Vinik, A. I., Nevoret, M., Casellini, C. & Parson, H. Neurovascular function and sudorimetry in health and disease. Curr. Diab. Rep. 13, 517–532 (2013).
Heikenfeld, J. et al. Accessing analytes in biofluids for peripheral biochemical monitoring. Nat. Biotechnol. 37, 407–419 (2019).
Baker, L. B. & Wolfe, A. S. Physiological mechanisms determining eccrine sweat composition. Eur. J. Appl. Physiol. 120, 719–752 (2020).
LeGrys, V., Briscoe, D. & McColley, S. Sweat Testing: Specimen Collection and Quantitative Chloride Analysis; Approved Guideline 4th edn (Clinical and Laboratory Standards Institute, 2019).
Hussain, J. N., Mantri, N. & Cohen, M. M. Working up a good sweat — the challenges of standardising sweat collection for metabolomics analysis. Clin. Biochem. Rev. 38, 13–34 (2017).
Cizza, G. et al. Elevated neuroimmune biomarkers in sweat patches and plasma of premenopausal women with major depressive disorder in remission: the POWER Study. Biol. Psychiatry 64, 907–911 (2008).
Sempionatto, J. R., Moon, J.-M. & Wang, J. Touch-based fingertip blood-free reliable glucose monitoring: personalized data processing for predicting blood glucose concentrations. ACS Sens. 6, 1875–1883 (2021).
Torrente-Rodríguez, R. M. et al. Investigation of cortisol dynamics in human sweat using a graphene-based wireless mHealth system. Matter 2, 921–937 (2020).
Busch, R. On the history of cystic fibrosis. Acta Univ. Carol. Med. 36, 13–15 (1990).
Pérez-Frías, J. et al. The history of cystic fibrosis. Open J. Pediatr. Child Health 4, 001–006 (2019).
Quinton, P. M. Physiological basis of cystic fibrosis: a historical perspective. Physiol. Rev. 79, S3–S22 (1999).
Darling, R. C., Disant’agnese, P. A., Perera, G. A. & Andersen, D. H. Electrolyte abnormalities of the sweat in fibrocystic disease of the pancreas. Am. J. Med. Sci. 225, 67–70 (1953).
Barbero, G. J., Kim, I. C. & Mcgavran, J. A simplified technique for the sweat test in the diagnosis of fibrocystic disease of the pancreas. Pediatrics 18, 189–192 (1956).
Gibson, E. & Cooke, E. A test for concentration of electrolytes in sweat in cystic fibrosis of the pancreas utilizing pilocarpine by iontophoresis. Pediatrics 23, 545–549 (1959).
Webster, H. L. & Rundell, C. A. Laboratory diagnosis of cystic fibrosis. Crit. Rev. Clin. Lab. Sci. 18, 313–338 (1982).
Sato, K. in Reviews of Physiology, Biochemistry and Pharmacology Vol. 79 (eds Adrian, R. H. et al.) 51–131 (Springer, 1977).
Sato, K., Feibleman, C. & Dobson, R. L. The electrolyte composition of pharmacologically and thermally stimulated sweat: a comparative study. J. Invest. Dermatol. 55, 433–438 (1970).
Sato, K. & Dobson, R. L. Regional and individual variations in the function of the human eccrine sweat gland. J. Invest. Dermatol. 54, 443–449 (1970).
Sato, K. Sweat induction from an isolated eccrine sweat gland. Am. J. Physiol. 225, 1147–1152 (1973).
Drexelius, A., Fehr, D., Vescoli, V., Heikenfeld, J. & Bonmarin, M. A simple non-contact optical method to quantify in-vivo sweat gland activity and pulsation. In IEEE Transactions on Biomedical Engineering 2638–2645 (IEEE, 2022).
Yanagawa, S., Yokozeki, H. & Sato, K. Origin of periodic acid–Schiff-reactive glycoprotein in human eccrine sweat. J. Appl. Physiol. 60, 1615–1622 (1986).
Nicolaidis, S. & Sivadjian, J. High-frequency pulsatile discharge of human sweat glands: myoepithelial mechanism. J. Appl. Physiol. 32, 86–90 (1972).
Ogawa, T. & Sugenoya, J. Pulsatile sweating and sympathetic sudomotor activity. Jpn. J. Physiol. 43, 275–289 (1993).
Schwartz, I. L. & Thaysen, J. H. Excretion of sodium and potassium in human sweat. J. Clin. Invest. 35, 114–120 (1956).
Baker, L. B. Physiology of sweat gland function: the roles of sweating and sweat composition in human health. Temperature 6, 211–259 (2019).
Quinton, P. M. Cystic fibrosis: lessons from the sweat gland. Physiology 22, 212–225 (2007).
Nadel, E. R. Control of sweating rate while exercising in the heat. Med. Sci. Sports 11, 31–35 (1979).
Nadel, E. R., Bullard, R. W. & Stolwijk, J. A. Importance of skin temperature in the regulation of sweating. J. Appl. Physiol. 31, 80–87 (1971).
Shibasaki, M. & Crandall, C. G. Mechanisms and controllers of eccrine sweating in humans. Front. Biosci. (Schol. Ed.) 2, 685–696 (2010).
Shibasaki, M., Secher, N. H., Selmer, C., Kondo, N. & Crandall, C. G. Central command is capable of modulating sweating from non-glabrous human skin. J. Physiol. 553, 999–1004 (2003).
Hu, Y., Converse, C., Lyons, M. C. & Hsu, W. H. Neural control of sweat secretion: a review. Br. J. Dermatol. 178, 1246–1256 (2018).
Simmers, P., Li, S. K., Kasting, G. & Heikenfeld, J. Prolonged and localized sweat stimulation by iontophoretic delivery of the slowly-metabolized cholinergic agent carbachol. J. Dermatol. Sci. 89, 40–51 (2018).
Souza, S. L., Graça, G. & Oliva, A. Characterization of sweat induced with pilocarpine, physical exercise, and collected passively by metabolomic analysis. Skin Res. Technol. 24, 187–195 (2018).
Sato, K., Kang, W. H., Saga, K. & Sato, K. T. Biology of sweat glands and their disorders. I. Normal sweat gland function. J. Am. Acad. Dermatol. 20, 537–563 (1989).
Sonner, Z. et al. The microfluidics of the eccrine sweat gland, including biomarker partitioning, transport, and biosensing implications. Biomicrofluidics 9, 031301 (2015).
Sato, F., Takemura, T., Hibino, T. & Sato, K. Lectin binding glycoproteins in human eccrine sweat. J. Invest. Dermatol. 88, 515–515 (1987).
Macroduct Sweat Collection System (Model 3700) Instruction/Service Manual (Wescor, 2004).
Huestis, M. A. et al. Sweat testing for cocaine, codeine and metabolites by gas chromatography–mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 733, 247–264 (1999).
Brueck, A., Iftekhar, T., Stannard, A. B., Yelamarthi, K. & Kaya, T. A real-time wireless sweat rate measurement system for physical activity monitoring. Sensors 18, 533 (2018).
Katchman, B. A., Zhu, M., Blain Christen, J. & Anderson, K. S. Eccrine sweat as a biofluid for profiling immune biomarkers. Proteomics Clin. Appl. 12, 1800010 (2018).
Matzeu, G., Fay, C., Vaillant, A., Coyle, S. & Diamond, D. A wearable device for monitoring sweat rates via image analysis. IEEE Trans. Biomed. Eng. 63, 1672–1680 (2016).
Mayaudon, H., Miloche, P.-O. & Bauduceau, B. A new simple method for assessing sudomotor function: relevance in type 2 diabetes. Diabetes Metab. 36, 450–454 (2010).
Baker, L. B. et al. Skin-interfaced microfluidic system with personalized sweating rate and sweat chloride analytics for sports science applications. Sci. Adv. 6, eabe3929 (2020).
Baker, L. B. et al. Sweating rate and sweat chloride concentration of elite male basketball players measured with a wearable microfluidic device versus the standard absorbent patch method. Int. J. Sport Nutr. Exerc. Metab. 1, 342–349 (2022).
Jia, W. et al. Electrochemical tattoo biosensors for real-time noninvasive lactate monitoring in human perspiration. Anal. Chem. 85, 6553–6560 (2013).
Guinovart, T. J., Bandodkar, A. R., Windmiller, J. J., Andrade, F. & Wang, J. A potentiometric tattoo sensor for monitoring ammonium in sweat. Analyst 138, 7031–7038 (2013).
Bandodkar, A. J. et al. Epidermal tattoo potentiometric sodium sensors with wireless signal transduction for continuous non-invasive sweat monitoring. Biosens. Bioelectron. 54, 603–609 (2014).
Huang, X. et al. Stretchable, wireless sensors and functional substrates for epidermal characterization of sweat. Small 10, 3083–3090 (2014).
Rose, D. P. et al. Adhesive RFID sensor patch for monitoring of sweat electrolytes. IEEE Trans. Biomed. Eng. 62, 1457–1465 (2015).
Glennon, T. et al. ‘SWEATCH’: a wearable platform for harvesting and analysing sweat sodium content. Electroanalysis 28, 1283–1289 (2016).
Gao, W. et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514 (2016).
Koh, A. et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 8, 366ra165 (2016).
Choi, J., Kang, D., Han, S., Kim, S. B. & Rogers, J. A. Thin, soft, skin-mounted microfluidic networks with capillary bursting valves for chrono-sampling of sweat. Adv. Healthc. Mater. 6, 1601355 (2017).
Nyein, H. Y. Y. et al. A wearable microfluidic sensing patch for dynamic sweat secretion analysis. ACS Sens. 3, 944–952 (2018).
Hauke, A. et al. Complete validation of a continuous and blood-correlated sweat biosensing device with integrated sweat stimulation. Lab Chip 18, 3750–3759 (2018).
Nyein, H. Y. Y. et al. A wearable patch for continuous analysis of thermoregulatory sweat at rest. Nat. Commun. 12, 1823 (2021).
Tai, L.-C. et al. Methylxanthine drug monitoring with wearable sweat sensors. Adv. Mater. 30, 1707442 (2018).
Tai, L.-C. et al. Wearable sweat band for noninvasive levodopa monitoring. Nano Lett. 19, 6346–6351 (2019).
Ruwe, T. Diverse drug classes partition into human sweat: implications for both sweat fundamentals and for therapeutic drug monitoring. Ther. Drug Monit. 10.1097/FTD.0000000000001110 (2023).
Harshman, S. W. et al. The proteomic and metabolomic characterization of exercise-induced sweat for human performance monitoring: a pilot investigation. PLoS ONE 13, e0203133 (2018).
Kwon, K. et al. An on-skin platform for wireless monitoring of flow rate, cumulative loss and temperature of sweat in real time. Nat. Electron. 4, 302–312 (2021).
Bandodkar, A. J. et al. Battery-free, skin-interfaced microfluidic/electronic systems for simultaneous electrochemical, colorimetric, and volumetric analysis of sweat. Sci. Adv. 5, eaav3294 (2019).
Heikenfeld, J. Non-invasive analyte access and sensing through eccrine sweat: challenges and outlook circa 2016. Electroanalysis 28, 1242–1249 (2016).
Moyen, N. E. et al. Accuracy of algorithm to non-invasively predict core body temperature using the Kenzen wearable device. Int. J. Environ. Res. Public Health 18, 13126 (2021).
Tang, W. et al. Touch-based stressless cortisol sensing. Adv. Mater. 33, 2008465 (2021).
Lin, S. et al. Natural perspiration sampling and in situ electrochemical analysis with hydrogel micropatches for user-identifiable and wireless chemo/biosensing. ACS Sens. 5, 93–102 (2020).
Paul, B., Demuru, S., Lafaye, C., Saubade, M. & Briand, D. Printed iontophoretic-integrated wearable microfluidic sweat-sensing patch for on-demand point-of-care sweat analysis. Adv. Mater. Technol. 6, 2000910 (2021).
Sonner, Z., Wilder, E., Gaillard, T., Kasting, G. & Heikenfeld, J. Integrated sudomotor axon reflex sweat stimulation for continuous sweat analyte analysis with individuals at rest. Lab Chip 17, 2550–2560 (2017).
Peng, R. et al. A new oil/membrane approach for integrated sweat sampling and sensing: sample volumes reduced from μL’s to nL’s and reduction of analyte contamination from skin. Lab Chip 16, 4415–4423 (2016).
Reeder, J. T. et al. Waterproof, electronics-enabled, epidermal microfluidic devices for sweat collection, biomarker analysis, and thermography in aquatic settings. Sci. Adv. 5, eaau6356 (2019).
Brebner, D. F. & Kerslake, D. McK. The time course of the decline in sweating produced by wetting the skin. J. Physiol. 175, 295–302 (1964).
Twine, N. B. et al. Open nanofluidic films with rapid transport and no analyte exchange for ultra-low sample volumes. Lab Chip 18, 2816–2825 (2018).
Baker, L. B. Sweating rate and sweat sodium concentration in athletes: a review of methodology and intra/interindividual variability. Sports Med. 47, 111–128 (2017).
Yuan, Z. et al. A multi-modal sweat sensing patch for cross-verification of sweat rate, total ionic charge, and Na+ concentration. Lab Chip 19, 3179–3189 (2019).
Wang, S. et al. An unconventional vertical fluidic-controlled wearable platform for synchronously detecting sweat rate and electrolyte concentration. Biosens. Bioelectron. 210, 114351 (2022).
Montain, S. J., Latzka, W. A. & Sawka, M. N. Control of thermoregulatory sweating is altered by hydration level and exercise intensity. J. Appl. Physiol. 79, 1434–1439 (1995).
Sawka, M. N. & Montain, S. J. Fluid and electrolyte supplementation for exercise heat stress. Am. J. Clin. Nutr. 72, 564S–572S (2000).
Nyein, H. Y. Y. et al. Regional and correlative sweat analysis using high-throughput microfluidic sensing patches toward decoding sweat. Sci. Adv. 5, eaaw9906 (2019).
Zhao, F. J. et al. Ultra-simple wearable local sweat volume monitoring patch based on swellable hydrogels. Lab Chip 20, 168–174 (2019).
Doolittle, J., Walker, P., Mills, T. & Thurston, J. Hyperhidrosis: an update on prevalence and severity in the United States. Arch. Dermatol. Res. 308, 743–749 (2016).
Korpelainen, J. T., Sotaniemi, K. A. & Myllylä, V. V. Asymmetric sweating in stroke: a prospective quantitative study of patients with hemispheral brain infarction. Neurology 43, 1211–1214 (1993).
Foster, K. G., Hey, E. N. & O’Connell, B. Sweat function in babies with defects of the central nervous system. Dev. Med. Child Neurol. 11, 94 (2008).
Cheshire, W. P. & Freeman, R. Disorders of sweating. Semin. Neurol. 23, 399–406 (2003).
Harker, M. Psychological sweating: a systematic review focused on aetiology and cutaneous response. Skin Pharmacol. Physiol. 26, 92–100 (2013).
Berglund, L. G. Comfort and humidity. ASHRAE J. 40, 35–41 (1998).
Rousseau, C. R. & Bühlmann, P. Calibration-free potentiometric sensing with solid-contact ion-selective electrodes. TrAC Trends Anal. Chem. 140, 116277 (2021).
Bhide, A., Muthukumar, S., Saini, A. & Prasad, S. Simultaneous lancet-free monitoring of alcohol and glucose from low-volumes of perspired human sweat. Sci. Rep. 8, 6507 (2018).
Arroyo-Currás, N., Dauphin-Ducharme, P., Scida, K. & Chávez, J. L. From the beaker to the body: translational challenges for electrochemical, aptamer-based sensors. Anal. Methods 12, 1288–1310 (2020).
Potyrailo, R. A., Conrad, R. C., Ellington, A. D. & Hieftje, G. M. Adapting selected nucleic acid ligands (aptamers) to biosensors. Anal. Chem. 70, 3419–3425 (1998).
Zhang, F., Xue, J., Shao, J. & Jia, L. Compilation of 222 drugs’ plasma protein binding data and guidance for study designs. Drug Discov. Today 17, 475–485 (2012).
Yuan, Y. et al. Oil-membrane protection of electrochemical sensors for fouling- and pH-insensitive detection of lipophilic analytes. ACS Appl. Mater. Interfaces 13, 53553–53563 (2021).
Shaver, A., Curtis, S. D. & Arroyo-Currás, N. Alkanethiol monolayer end groups affect the long-term operational stability and signaling of electrochemical, aptamer-based sensors in biological fluids. ACS Appl. Mater. Interfaces 12, 11214–11223 (2020).
Watkins, Z., Karajić, A., Young, T., White, R. & Heikenfeld, J. Week-long operation of electrochemical aptamer sensors: new insights into self-assembled monolayer degradation mechanisms and solutions for stability in biofluid at body temperature. ACS Sens. 8, 1119–1131 (2023).
Xu, J. & Lee, H. Anti-biofouling strategies for long-term continuous use of implantable biosensors. Chemosensors 8, 66 (2020).
Li, H., Dauphin-Ducharme, P., Ortega, G. & Plaxco, K. W. Calibration-free electrochemical biosensors supporting accurate molecular measurements directly in undiluted whole blood. J. Am. Chem. Soc. 139, 11207–11213 (2017).
Das, S. K., Nayak, K. K., Krishnaswamy, P. R., Kumar, V. & Bhat, N. Review—electrochemistry and other emerging technologies for continuous glucose monitoring devices. ECS Sens. Plus 1, 031601 (2022).
Troudt, B. K., Rousseau, C. R., Dong, X. I. N., Anderson, E. L. & Bühlmann, P. Recent progress in the development of improved reference electrodes for electrochemistry. Anal. Sci. 38, 71–83 (2022).
Pirovano, P. et al. A wearable sensor for the detection of sodium and potassium in human sweat during exercise. Talanta 219, 121145 (2020).
Forlenza, G. P., Kushner, T., Messer, L. H., Wadwa, R. P. & Sankaranarayanan, S. Factory-calibrated continuous glucose monitoring: how and why it works, and the dangers of reuse beyond approved duration of wear. Diabetes Technol. Ther. 21, 222–229 (2019).
Dautta, M. et al. Tape-free, digital wearable band for exercise sweat rate monitoring. Adv. Mater. Technol. 8, 2201187 (2023).
Emaminejad, S. et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl Acad. Sci. USA 114, 4625–4630 (2017).
Klous, L., de Ruiter, C. J., Scherrer, S., Gerrett, N. & Daanen, H. A. M. The (in)dependency of blood and sweat sodium, chloride, potassium, ammonia, lactate and glucose concentrations during submaximal exercise. Eur. J. Appl. Physiol. 121, 803–816 (2021).
Wiorek, A., Parrilla, M., Cuartero, M. & Crespo, G. A. Epidermal patch with glucose biosensor: pH and temperature correction toward more accurate sweat analysis during sport practice. Anal. Chem. 92, 10153–10161 (2020).
Francis, J., Stamper, I., Heikenfeld, J. & Gomez, E. F. Digital nanoliter to milliliter flow rate sensor with in vivo demonstration for continuous sweat rate measurement. Lab Chip 19, 178–185 (2019).
Moon, J.-M. et al. Non-invasive sweat-based tracking of l-dopa pharmacokinetic profiles following an oral tablet administration. Angew. Chem. Int. Ed. Engl. 133, 19222–19226 (2021).
Montanga, W., Kligman, A. M. & Carlisle, K. S. Atlas of Normal Human Skin (Springer, 1992).
Illigens, B. M. W. & Gibbons, C. H. in Handbook of Clinical Neurology (eds Levin, K. H. & Chauvel, P.) Vol. 160, 419–433 (Elsevier, 2019).
Acknowledgements
A.J. acknowledges support from the NSF NASCENT Center, the Berkeley Sensors and Actuators Center and the Bakar Fellowship. J.H. acknowledges support from the US Air Force Office of Scientific Research (USAF contract no. FA9550-20-1-0117), a the National Science Foundation CBET award (2125056) and a National Science Foundation ECCS award (2025720). C.M. acknowledges support from the US Air Force Office of Scientific Research (USAF contract no. FA955-20-1-0117-Sub 013176-002), the National Institutes of Health (NIDDK R21/33 DK128711) and the Ross Mosier Cystic Fibrosis Research Laboratories Gift Fund. N.D. acknowledges support from the National Defense Science and Engineering Graduate Fellowship Program.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
J.H. has multiple patents that have been licensed to entities pursuing commercialization of sweat biosensing devices.
Peer review information
Nature Biotechnology thanks Chwee Teck Lim, **ghua Li and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Fig. 1 and Table 1
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Davis, N., Heikenfeld, J., Milla, C. et al. The challenges and promise of sweat sensing. Nat Biotechnol 42, 860–871 (2024). https://doi.org/10.1038/s41587-023-02059-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41587-023-02059-1
- Springer Nature America, Inc.