Abstract
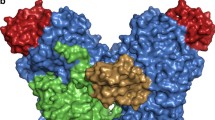
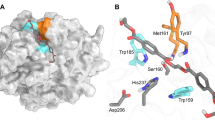
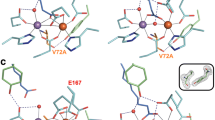
Membrane-bound styrene oxide isomerase (SOI) catalyses the Meinwald rearrangement—a Lewis-acid-catalysed isomerization of an epoxide to a carbonyl compound—and has been used in single and cascade reactions. However, the structural information that explains its reaction mechanism has remained elusive. Here we determine cryo-electron microscopy (cryo-EM) structures of SOI bound to a single-domain antibody with and without the competitive inhibitor benzylamine, and elucidate the catalytic mechanism using electron paramagnetic resonance spectroscopy, functional assays, biophysical methods and docking experiments. We find ferric haem b bound at the subunit interface of the trimeric enzyme through H58, where Fe(III) acts as the Lewis acid by binding to the epoxide oxygen. Y103 and N64 and a hydrophobic pocket binding the oxygen of the epoxide and the aryl group, respectively, position substrates in a manner that explains the high regio-selectivity and stereo-specificity of SOI. Our findings can support extending the range of epoxide substrates and be used to potentially repurpose SOI for the catalysis of new-to-nature Fe-based chemical reactions.

Similar content being viewed by others
Main
Epoxides are highly versatile building blocks for the synthesis of many organic molecules. In the presence of strong Lewis or Brønsted acids, epoxides isomerize to carbonyl compounds by Meinwald rearrangement1, which generates multifunctional active aldehyde and ketone intermediates that are widely applied in fine chemical and pharmaceutical syntheses. However, this chemical reaction usually requires the use of corrosive acids as catalysts and protective anhydrous and inert atmospheric conditions, as the substrates are moisture- and air-sensitive2. Furthermore, the Meinwald rearrangement suffers from low yields due to side reactions and poor regio-selectivity and stereo-specificity, typically resulting in the formation of a mixture of compounds3.
In contrast, styrene oxide isomerase (SOI), an integral membrane protein found in the styrene-degradation pathway of microbes4,5,6,7, catalyses the isomerization of aryl epoxides such as styrene oxide derivatives to carbonyl compounds under physiological conditions by Meinwald rearrangement. SOI possesses several characteristics that make it an attractive enzyme for biocatalytic applications and an alternative to chemical synthesis. First, there are very few unique enzymes that can catalyse the isomerization of an epoxide to an aldehyde. Apart from SOI8,9 and quinolone epoxide rearrangement protein (PenF)10 from the penigequinolone synthesis pathway, no other enzymes have been reported to catalyse Meinwald rearrangements. Second, SOI has a broad substrate range and may therefore be used for the production of a variety of organic molecules, including new compounds (Extended Data Fig. 1a) for synthetic applications5). The R enantiomer has been shown to react more quickly, but the difference is too small to be rationalized by static structural considerations. Similarly, the ring-opened sp2-carbocations of both enantiomers, assumed to be stabilized by co-planarity with the aryl ring and with their oxygens still ligated to the haem iron, can be modelled without causing substantial repulsive interactions and maintaining the approximate mirror relationship. Fixed in this relatively rigid conformation, only one of the two hydrogens of Cβ of the epoxide is in a favourable position to shift to Cα, explaining the high specificity of the reaction and the conserved chirality at Cα. At the same time, it explains why internal epoxides methylated at Cβ, such as (1R,2R)-2-methyl-3-phenyloxirane, gave only one diastereomer by shifting the methyl group in the trans-position, but not the other product by transferring the hydrogen in the cis-positionIdentification of the ferric haem b prosthetic group XANES data for SOI was collected at the Swiss Light Source at beamline SuperXAS, using a Si(111) monochromator, Si mirrors for collimation and harmonic rejection, a toroidal Rh-coated mirror for focusing, ionization chambers for incident intensity detection and a five-element silicon drift detector (SDD) for X-ray fluorescence measurements. A 200–300-μl sample of 3 mg ml−1 SOI was filled into a 4–5-cm kapton capillary (diameter of 2 mm) using a Hamilton syringe. The following measurement strategy was used. First, a series of XANES spectra were measured to check the influence of X-ray-induced damage at room temperature After that, the sample was translated to fresh sections multiple times, and scans were performed during the time when the X-ray-induced damage was small. Measured XANES spectra were compared with theoretical XANES calculations performed using finite-difference method near-edge structure (FDMNES) code using the full multiple scattering method38. Our data show a mixture of 5- and 6-coordinated SOI at room temperature in solution (Extended Data Fig. 2). The fifth axial ligand of iron is a nitrogen atom of histidine with ~2.3 Å and the sixth distal ligand of iron can be an oxygen or nitrogen atom. The EPR samples contained 305 µM SOI, 545 µM SOI–NB complex or 309 µM SOI Y103A in 200 µl of buffer consisting of 20 mM HEPES, 150 mM NaCl and 0.03% DDM pH 7.5, unless stated otherwise. Reduced samples were prepared by addition of 10 mM sodium dithionite in an anaerobic glovebox. Stock solutions of 200 mM styrene oxide and phenylacetaldehyde were prepared in methanol. A 200 mM benzylamine stock solution was prepared in water. Styrene oxide, phenylacetaldehyde and benzylamine were added to a final concentration of 10 mM to the three different SOI samples, and the EPR samples were frozen in liquid nitrogen. EPR spectra were recorded on a Bruker EMXplus spectrometer with a helium-flow cryostat at 17 K (refs. 39,40) using the following EPR parameters: microwave frequency of 9.410 GHz, microwave power of 20 mW, modulation frequency of 100 kHz and a modulation amplitude of 20 G. The magnetic field was calibrated using the Bruker BDPA (1,3-bis(diphenylene)-2-phenylallyl radical) standard with a g-value of 2.00254 ± 0.00003. For isomerization activity measurements, the following coupled enzyme assay was carried out (Extended Data Fig. 5c). In a 1.5-ml cuvette, a 1-ml reaction for (S)-styrene oxide was performed at 25 °C in reaction buffer (0.05 M potassium phosphate buffer pH 8, 0.01% DDM) containing 2 mM NAD+ and 6 U of EcALDH. The change in absorbance at 340 nm was recorded at regular time intervals using a Hitachi U2900 spectrophotometer, and absorbance units were converted to concentration using the extinction coefficient at 340 nm (ɛ340 nm) value of NADH (6.22 Abs mM−1 cm−1). The slope of the concentration–time plot for the first 15 s was calculated to determine activity, with 1 U defined as the activity of SOI that gives 1 µmol of product in 1 min under assay conditions. To determine the kinetic parameters KM and kcat, the isomerization activity was determined with (S)-styrene oxide concentrations of 0.1–5.0 mM. SOI (0.14 μg, 7.26 pmol) or 0.07 μg (3.63 pmol) of SOI–NB complex (containing an equimolar mixture of SOI and nanobody) was added to the assay mixture at 25 °C in reaction buffer (0.05 M potassium phosphate buffer, pH 8, 0.01% DDM) containing 2 mM NAD+, 6 U EcALDH, to start the reaction. The slope of the concentration–time plot for the first 15 s was used to determine the initial rate of each reaction in mM min−1, to obtain a relationship between initial activity and concentration. Each reaction was performed in triplicate. AlphaFold2 (ref. 22) was implemented by locally running an adapted code written by ColabFold41. All runs used only the sequence of SOI, with no templates or Amber relaxation. We assessed monomeric to tetrameric models. The lipid nanodisc reconstitution of SOI was performed using E. coli polar lipids and an MSP1D1 nanodisc. The concentrations of SOI for nanodisc reconstitution were in the 300–400 μM range, with a molar ratio of SOI to MSP1D1 to lipid of 3:2:75. Initially, 5 mg of E. coli polar lipid (EPL, Avanti) was dissolved in 100 μl of chloroform and dried under a stream of N2. The resulting lipid film was then mixed with 600 μl of buffer (50 mM Tris-HCl pH 8.0, 150 mM NaCl and 1% DDM), and sonicated in a bath sonicator (Bandelin SONOREX SUPER) until the mixture turned translucent. The detergent-solubilized EPL extract was combined with freshly purified SOI (in a 1:25 molar ratio), then gently mixed for 30 min at room temperature. Following this incubation, MSP1D1 was introduced to the protein–lipid mixture and incubated at room temperature for another 30 min. Incorporation of SOI in nanodisc was triggered by adding 300 mg of wet Bio-beads (precleaned with 100% methanol and Milli-Q water). The reconstitution solution was then incubated overnight at 4 °C with gentle mixing. The supernatant was cleared of beads, and the sample was spun before loading onto a Superdex 200 Increase 10/300 GL column (GE Healthcare) equilibrated in 20 mM Tris pH 8.0 and 150 mM NaCl. The peak fractions corresponding to SOI in MSP1D1 (elution volume, 10.3–11.8 ml) were collected, concentrated with a 100-kDa-cutoff Amicon concentrator (Millipore) and used for cryo-EM grid preparation. The cryo-EM samples of SOI–NB were prepared using freshly reconstituted SOI–EPL–MSP1D1 nanodisc complex. SOI–benzylamine was prepared by adding 1 mM benzylamine (100 mM stock concentration in 100% DMSO) to SOI–NB, then incubating the samples on ice for 10–15 min. The final concentration of SOI–NB and SOI–NB–BA complexes used for freezing grids was ~5–7 mg ml−1. The concentration of protein was estimated based on absorbance at 280 nm and a normalized extinction coefficient of 154,685 M−1 cm−1 (considering one molecule of MSP1D1 per three molecules of SOI and nanobody molecule, respectively). All the grids used for cryo-EM grid preparation were freshly glow-discharged in a PELCO easiGlow (Ted Pella) glow-discharge cleaning system for 25 s at 30 mA in air, then 3.5 μl of protein solution was applied to Quantifoil 1.2/1.3 grids (300 mesh), blotted for 3 s with blot force 20, and plunged into liquid ethane using a Vitrobot Mark IV system (Thermo Fisher Scientific) with 100% humidity and an ambient temperature of 4 °C. The frozen grids were stored in liquid nitrogen for subsequent cryo-EM data collection. All the cryo-EM datasets of SOI were collected using EPU software on a 300-kV Titan Krios system (Thermo Fisher Scientific) equipped with a Gatan K3 direct electron detector and a Gatan Quantum-LS GIF, at ScopeM, ETH Zurich. All movies were acquired in super-resolution mode with a defocus range of −0.5 to −3 μm and were binned twofold after acquisition in EPU. The dataset of SOI apo was composed of 8,942 movies with an average dose of 65 e−/Å2 and final pixel size of 0.66 Å. The cryo-EM processing was performed in Relion (version 3.1.3 and 4.0.0)42,43. A flow chart of cryo-EM processing of the SOI–NB complex is provided in Extended Data Fig. 6. In brief, all movie stacks were motion-corrected using MotionCorr2 (version 1.4.0)44, then CTF-corrected using Gctf (version 1.0.6)45. A total of 2,715,264 particles were autopicked, then subjected to several rounds of 2D classifications, yielding 955,410 particles. The best set of 2D classes was then used to generate an initial model to be used in 3D classification. After multiple rounds of 2D and 3D clarifications, a set of 396,760 particles were selected for masked 3D refinement (using masks excluding the nanodisc density), resulting in a 3D reconstruction at a resolution of 2.54 Å. These refined particles were then subjected to CTF refinement and particle polishing, and another round of 3D classification without alignment and with masking of the nanodisc. The particles from the best 3D class were subjected to several iterative cycles of 3D refinement, CTF refinement and particle polishing, yielding a final post-processed density map at a resolution of 2.05 Å. For the SOI–NB–BA complex, 11,905 movies with 56 e−/Å2 were collected. The data-collection strategy and image processing of the SOI–NB–BA complex were similar to those used for the SOI–NB complex. The 3D projections from the best 3D class from SOI apo were used as templates for autopicking and 3D classification jobs. The detailed steps for processing of the SOI–NB–BA complex are shown in Extended Data Fig. 7. The cryo-EM density features of the transmembrane helices of SOI, nanobodies and ferric haem b are shown in Extended Data Fig. 8. The resolutions of the cryo-EM maps of the SOI–NB complex and SOI–NB–BA complex are shown by Fourier shell correlation (FSC) curves (Supplementary Fig. 1). Details of cryo-EM data collection and the statistical analysis are shown in Table 1. As starting models, the AlphaFold22 predicated model of SOI and a homology model of the nanobody generated using SwissModel46 (with PDB 5VAK as template) were docked on the final post-processed density map and manually refined using Coot47. The ligands, the ferric haem b prosthetic group and benzylamine were generated from SMILE codes using the eLBOW program in Phenix48. The structures were finally refined using phenix.real_space_refine48. The quality of the final models was assessed using MolProbity49. Local resolution maps were calculated by ResMap50 implemented in Relion 4.0.0 (ref. 42). All figures were generated using PyMOL 2.5.2 (ref. 51) and ChimeraX52. Further information on research design is available in the Nature Portfolio Reporting Summary linked to this Article.EPR spectroscopy
Functional characterization
AlphaFold structure prediction
Reconstitution of SOI in a lipid nanodisc
Cryo-EM sample preparation and data collection
Cryo-EM data collection and processing
Model building and refinement
Reporting summary
Data availability
Data supporting the findings of this study are available within the main Article, including Extended Data, Supplementary Information and source data files. Further details and raw data from in silico docking are also available from the corresponding authors upon request. The atomic coordinates and EM density maps of the SOI–NB complex and SOI–NB–BA complex are deposited in the Worldwide Protein Data Bank (wwPDB) and Electron Microscopy Data Bank (EMDB) under the respective accession codes 8PNV/EMD-17786 and 8PNU/EMD-17785. Source data are provided with this paper.
References
Meinwald, J. S., Singhcha, M. & Labana, S. S. Peracid reactions. III. Oxidation of bicyclo[2.2.1]heptadiene. J. Am. Chem. Soc. 85, 582 (1963).
Karamé, I., Tommasino, M. L. & Lemaire, M. Iridium-catalyzed alternative of the Meinwald rearrangement. Tetrahedron Lett. 44, 7687–7689 (2003).
Ranu, B. C. & Jana, U. Indium(III) chloride-promoted rearrangement of epoxides: a selective synthesis of substituted benzylic aldehydes and ketones. J. Org. Chem. 63, 8212–8216 (1998).
Panke, S., Witholt, B., Schmid, A. & Wubbolts, M. G. Towards a biocatalyst for (S)-styrene oxide production: characterization of the styrene degradation pathway of Pseudomonas sp. strain VLB120. Appl. Environ. Microbiol. 64, 2032–2043 (1998).
Itoh, N., Hayashi, K., Okada, K., Ito, T. & Mizuguchi, N. Characterization of styrene oxide isomerase, a key enzyme of styrene and styrene oxide metabolism in Corynebacterium sp. Biosci. Biotechnol. Biochem. 61, 2058–2062 (1997).
Hartmans, C. Na. S. Formation and degradation of styrene oxide stereoisomers by different microorganisms. Biocatalysis 10, 219–225 (1994).
Hartmans, S., Smits, J. P., van der Werf, M. J., Volkering, F. & de Bont, J. A. Metabolism of styrene oxide and 2-phenylethanol in the styrene-degrading Xanthobacter strain 124X. Appl. Environ. Microbiol. 55, 2850–2855 (1989).
See, W. W. L. & Li, Z. Styrene oxide isomerase-catalyzed Meinwald rearrangement in cascade biotransformations: synthesis of chiral and/or natural chemicals. Chemistry 29, e202300102 (2023).
Choo, J. P. S. & Li, Z. Styrene oxide isomerase catalyzed Meinwald rearrangement reaction: discovery and application in single-step and one-pot cascade reactions. Org. Process Res. Dev. 26, 1960–1970 (2022).
Zou, Y. et al. Enzyme-catalyzed cationic epoxide rearrangements in quinolone alkaloid biosynthesis-PenF. Nat. Chem. Biol. 13, 325–332 (2017).
**n, R. P., See, W. W. L., Yun, H., Li, X. R. & Li, Z. Enzyme-catalyzed Meinwald rearrangement with an unusual regioselective and stereospecific 1,2-methyl shift. Angew. Chem. Int. Ed. 61, e202204889 (2022).
Sekar, B. S., Lukito, B. R. & Li, Z. Production of natural 2-phenylethanol from glucose or glycerol with coupled Escherichia coli strains expressing l-phenylalanine biosynthesis pathway and artificial biocascades. ACS Sustain. Chem. Eng. https://doi.org/10.1021/acssuschemeng.9b01569 (2019).
Lukito, B. R., Wu, S. K., Saw, H. J. J. & Li, Z. One-pot production of natural 2-phenylethanol from l-phenylalanine via cascade biotransformations. ChemCatChem 11, 831–840 (2019).
Wu, S., Zhou, Y., Seet, D. & Li, Z. Regio- and stereoselective oxidation of styrene derivatives to arylalkanoic acids via one-pot cascade biotransformations. Adv. Synth. Catal. 359, 2132–2141 (2017).
Wu, S., Liu, J. & Li, Z. Biocatalytic formal anti-Markovnikov hydroamination and hydration of aryl alkenes. ACS Catal. 7, 5225–5233 (2017).
Meza, A. et al. Efficient chemoenzymatic synthesis of α-aryl aldehydes as intermediates in C-C bond forming biocatalytic cascades. ACS Catal. 12, 10700–10710 (2022).
See, W. W. L., Li, X. R. & Li, Z. Biocatalytic cascade conversion of racemic epoxides to (S)-2-arylpropionic acids, (R)- and (S)-2-arylpropyl amines. Adv. Synth. Catal. 365, 68–77 (2023).
Choo, J. P. S., Kammerer, R. A., Li, X. & Li, Z. High‐level production of phenylacetaldehyde using fusion‐tagged styrene oxide isomerase. Adv. Synth. Catal. https://doi.org/10.1002/adsc.202001500 (2021).
Oelschlagel, M., Groning, J. A., Tischler, D., Kaschabek, S. R. & Schlomann, M. Styrene oxide isomerase of Rhodococcus opacus 1CP, a highly stable and considerably active enzyme. Appl. Environ. Microbiol. 78, 4330–4337 (2012).
Lau, S. Y., Taneja, A. K. & Hodges, R. S. Synthesis of a model protein of defined secondary and quaternary structure. Effect of chain length on the stabilization and formation of two-stranded alpha-helical coiled-coils. J. Biol. Chem. 259, 13253–13261 (1984).
Mehta, V. et al. Structure of Mycobacterium tuberculosis Cya, an evolutionary ancestor of the mammalian membrane adenylyl cyclases. eLife 11, e77032 (2022).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Perrakis, A. & Sixma, T. K. AI revolutions in biology: the joys and perils of AlphaFold. EMBO Rep. 22, e54046 (2021).
Kondo, H. X. & Takano, Y. Analysis of fluctuation in the heme-binding pocket and heme distortion in hemoglobin and myoglobin. Life (Basel) 12, 210 (2022).
Kondo, H. X., Kanematsu, Y. & Takano, Y. Structure of heme-binding pocket in heme protein is generally rigid and can be predicted by AlphaFold2. Chem. Lett. 51, 704–708 (2022).
Holm, L., Laiho, A., Toronen, P. & Salgado, M. DALI shines a light on remote homologs: one hundred discoveries. Protein Sci. 32, e4519 (2023).
Zoppellaro, G. et al. Review: studies of ferric heme proteins with highly anisotropic/highly axial low spin (S = 1/2) electron paramagnetic resonance signals with bis-histidine and histidine-methionine axial iron coordination. Biopolymers 91, 1064–1082 (2009).
Vries, S. D. & Albracht, S. P. J. Intensity of highly anisotropic low-spin heme EPR signals. Biochim. Biophys. Acta 546, 334–340 (1979).
Gadsby, P. M., Peterson, J., Foote, C., Greenwood, C. & Thomson, A. J. Identification of the ligand-exchange process in the alkaline transition of horse heart cytochrome c. Biochem. J. 246, 43–54 (1987).
Miyamoto, K., Okuro, K. & Ohta, H. Substrate specificity and reaction mechanism of recombinant styrene oxide isomerase from Pseudomonas putida S12. Tetrahedron Lett. 48, 3255–3257 (2007).
Coelho, P. S., Brustad, E. M., Kannan, A. & Arnold, F. H. Olefin cyclopropanation via carbene transfer catalyzed by engineered cytochrome P450 enzymes. Science 339, 307–310 (2013).
Zheng, L., Baumann, U. & Reymond, J. L. An efficient one-step site-directed and site-saturation mutagenesis protocol. Nucleic Acids Res. 32, e115 (2004).
Kariuki, C. K. & Magez, S. Improving the yield of recalcitrant Nanobodies® by simple modifications to the standard protocol. Protein Expr. Purif. 185, 105906 (2021).
Ritchie, T. K. et al. Reconstitution of membrane proteins in phospholipid bilayer nanodiscs. Methods Enzymol. 464, 211–231 (2009).
Geertsma, E. R. & Dutzler, R. A versatile and efficient high-throughput cloning tool for structural biology. Biochemistry 50, 3272–3278 (2011).
Pardon, E. et al. A general protocol for the generation of Nanobodies for structural biology. Nat. Protoc. 9, 674–693 (2014).
Ireland, S. M., Sula, A. & Wallace, B. A. Thermal melt circular dichroism spectroscopic studies for identifying stabilising amphipathic molecules for the voltage-gated sodium channel NavMs. Biopolymers 109, e23067 (2018).
Bunau, O. & Joly, Y. Self-consistent aspects of X-ray absorption calculations. J. Phys. Condens. Matter 21, 345501 (2009).
Salmeen, I. & Palmer, G. Electron paramagnetic resonance of beef-heart ferricytochrome C. J. Chem. Phys. 48, 2049–2052 (1968).
Lundin, A. & Aasa, R. A simple device to maintain temperatures in the range 4.2–100 K for EPR measurements. J. Magn. Reson. 8, 70–73 (1972).
Mirdita, M. et al. ColabFold: making protein folding accessible to all. Nat. Methods 19, 679–682 (2022).
Kimanius, D., Dong, L. Y., Sharov, G., Nakane, T. & Scheres, S. H. W. New tools for automated cryo-EM single-particle analysis in RELION-4.0. Biochem. J. 478, 4169–4185 (2021).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zhang, K. Gctf: real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Waterhouse, A. et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 46, W296–W303 (2018).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010).
Kucukelbir, A., Sigworth, F. J. & Tagare, H. D. Quantifying the local resolution of cryo-EM density maps. Nat. Methods 11, 63–65 (2014).
Schrödinger, L. & DeLano, W. PyMOL http://www.pymol.org/pymol (2020).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators and developers. Protein Sci. 30, 70–82 (2021).
Acknowledgements
This project was supported by a budget to the Protein Engineering group of the Laboratory of Biomolecular Research at Paul Scherrer Institute (PSI). PSI supported this project in its conceptualization, design, data collection, analysis, decision to publish and preparation of the manuscript. Part of this research (Z.L. and J.P.S.C.) was supported by the National Research Foundation (NRF), Singapore, through a Competitive Research Program (CRP) (project ID NRF-CRP17-2017-03), and the Ministry of Education, Singapore, through a MOE Tier 2 grant (project ID MOE 2016-T2-2-140) and an MOE Tier 1 grant (WBS: A-0009183-01-00). The funder supported conceptualization, data collection and analysis of this project.
Funding
Open Access funding provided by Lib4RI – Library for the Research Institutes within the ETH Domain: Eawag, Empa, PSI & WSL.
Author information
Authors and Affiliations
Contributions
X.L. and R.A.K. conceptualized the study. X.L. and R.A.K. designed the experiments. X.L. performed SOI and nanobody preparation and characterization, carried out EM sample preparation, EPR and kinetic studies and made illustrations. R.A.K. made all constructs used in this work and identified benzylamine. Z.L. established the initial SOI expression, activity assay and chemical reaction mechanism. B.K. reconstituted SOI into nanodiscs, prepared EM samples, collected and analysed EM data, determined the structures, analysed the structures, made most of the illustrations and deposited structures. J.P.S.C. performed SOI reaction assays, kinetic studies and data analysis, and made the reaction mechanism illustrations. P.-L.H. carried out EPR measurements, analysed and interpreted EPR data and made illustrations. G.S. performed XANES experiments and provided illustrations. S.S. produced nanobodies. S.K. and D.T. provided UV–vis spectra and SOI homologue constructs. F.K.W. analysed the structure and proposed mechanisms. V.M.K. collected and analysed EM data and provided illustrations. X.L. and R.A.K. wrote the paper with help from the other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Andrew Buller, Kai Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Summary of enzyme cascades involving SOI and the bacterial styrene-degradation pathway.
a, Isomerization of phenyl epoxides by membrane-bound SOI can be used to produce a variety of valuable compounds by involving other enzymes in cascade reactions. The high regio- and stereo-specificity of SOI is important for the synthesis of (S)- and (R)-configured alcohols, acids and amines. b, The styrene-degradation pathway comprises styrene monooxygenase (SMO), styrene oxide isomerase (SOI), and phenylacetaldehyde dehydrogenase (PAD). Styrene monooxygenases are two-component flavoproteins that catalyse the NADH and FAD-dependent enantioselective epoxidation of styrene to styrene oxide. Styrene oxide isomerase is a membrane-bound protein that catalyses the isomerization of styrene oxide to phenylacetaldehyde. Phenylacetaldehyde dehydrogenase catalyses the NAD+-dependent oxidation of phenylactealdehyde to phenylacetic acid. Phenylacetic acid catabolism leads to the production of succinyl-CoA and acetyl-CoA, which is then feed into the tricarboxylic acid (TCA) cycle.
Extended Data Fig. 2 Sequence alignment of SOI homologues.
Multiple sequence alignment of SOI homologues that were expressed was performed using the tool ClustalW with standard parameters in MEGA 7 (Version 7.0.26). Four Transmembrane helices were labelled. The amino acids were colour coded as follows, small non-polar (G,A) in orange; hydrophobic (V,I, L, P, F, Y, M, W) in green; polar (C, N, Q, H, S. T) in magenta; negatively charged (D, E) in red and positively charged (K, R) in blue.
Extended Data Fig. 3 Purification of recombinant SOI and characterization by size-exclusion chromatography and UV-Vis absorption.
a, Purified SOI showing the typical red color b, SDS–PAGE of purified SOI showing two bands migrating at 17 and 32 kDa that correspond to monomeric (calculated Mw = 19.7 kDa) and trimeric (calculated Mw = 59kDa) SOI. c, Homogenous peak of SOI after by Superdex 200 size-exclusion chromatography in the presence of 0.03% DDM. d, UV-Vis spectra showing a peak maximum at 421 nm. The spectrum shifted upon mixing with the reducing agent sodium dithionite from 421 nm to 419 nm and an additional peak emerged at 558 nm, indicating the presence of a reducible haem b prosthetic group.
Extended Data Fig. 4 CD analysis and SEC-MALS of SOI.
a, CD spectrum of SOI. b, Temperature-induced unfolding profile of purified SOI at 222nm. c, SEC-MALS analysis of SOI in 0.03% DDM. The analysis based on data from LS, UV, and dRI detectors revealed a molecular mass for SOI in DDM of 136 kDa.
Extended Data Fig. 5 Kinetic characterization of SOI in the presence of varying benzylamine concentrations.
a, Michaelis-Menten plot of specific isomerization activities of SOI (0.14 µg) towards (S)-styrene oxide in the presence of varying benzylamine concentrations of 0 mM (blue), 0.01 mM (orange) or 0.05 mM (dark orange). Curve fitting was performed by non-linear regression with MATLAB (solid line). Points corresponding to KM in the model are indicated with ✶. The data shown represent the averages of triplicate experimental results, with error bars indicating standard deviation. b, Lineweaver-Burke plot for the isomerization activity of SOI (0.14 µg) in the presence of varying benzylamine concentrations of 0 mM (blue), 0.01 mM (orange) or 0.05 mM (dark orange). One unit (U) of activity is defined as the formation of 1 µmol of phenylacetaldehyde from (S)-styrene oxide per min under assay conditions of 1 mL potassium phosphate buffer (50 mM, pH 8.0) at 25 °C. c, NADH-producing cascade with EcALDH for determination of SOI activity by coupled enzyme assay.
Extended Data Fig. 6 Cryo-EM data processing of SOI–NB complex.
a, Cryo-EM image processing scheme used for 3D reconstruction. b, Representative micrographs. c, Representative 2D classes. d, Angular distribution histogram of the refined map. e, Local resolution map.
Extended Data Fig. 7 Cryo-EM data processing of SOI–NB-BA complex.
a, Cryo-EM image processing scheme used for 3D reconstruction. b, Representative micrographs. c, Representative 2D classes. d, Angular distribution histogram of the refined map. e, Local resolution map.
Extended Data Fig. 8 Cryo-EM density features of SOI.
a, Isolated density map for four transmembrane (TM) helices and intracellular loops of SOI contoured at 12σ threshold level. b, Cryo-EM density features of nanobody contoured at 10σ levels. c-e, Cryo-EM density features of the haem b prosthetic group in the absence (panel c) and in the presence of the competitive inhibitor benzylamine (panel d), and density features of the benzylamine (panel e). f-h, Cryo-EM density features of overall SOI–NB complex showing densities features of ordered water molecules near the catalytic centre (panel g) and the SOI–NB interface (panel h).
Extended Data Fig. 9 EPR characterization of purified SOI, SOI–NB complex and the Y103A mutant.
a, EPR spectra of SOI WT. From top to bottom: purified enzyme, 10 mM sodium dithionite added, 10 mM styrene oxide added, 10 mM phenylacetaldehyde added, 10 mM benzylamine added. b, EPR spectra of SOI, SOI–NB complex and the Y103A mutant. The signal intensity of the different spectra has been normalized with the protein concentration.
Extended Data Fig. 10 X-ray absorption near-edge structure (XANES) characterization of SOI.
a, Theoretical XANES spectra for 5-coordinated and 6-coordinated models. Spectra are calculated with full multiple scattering theory for the cluster with a 5.5 Å radius using SOI–NB complex structure b, Comparison of theoretical spectrum calculated for 6-coordinated and 5-coordinated models in 1:1 ratio with the experimental XANES spectrum.
Supplementary information
Supplementary Information
Supplementary Table 1 and Figs. 1 and 2.
Supplementary Table 1
Source data for Supplementary Table 1.
Source data
Source Data Extended Data Fig. 2
Sequence alignment.
Source Data Extended Data Fig. 3
SDS-Gel, the chromatogram of SOI on Superdex 200 size-exclusion chromatography and UV-Vis spectra of SOI.
Source Data Extended Data Fig. 3c
SDS-Gel, the chromatogram of SOI on Superdex 200 size-exclusion chromatography and UV-Vis spectra of SOI.
Source Data Extended Data Fig. 3d
SDS-Gel, the chromatogram of SOI on Superdex 200 size-exclusion chromatography and UV-Vis spectra of SOI.
Source Data Extended Data Fig. 4
CD analysis and SEC-MALS analysis of SOI.
Source Data Extended Data Fig. 4c
CD analysis and SEC-MALS analysis of SOI.
Source Data Extended Data Fig. 5
Kinetic characterizations of SOI.
Source Data Extended Data Fig. 6
EPR characterization of SOI, SOI–NB complex and Y103A mutant.
Source Data Extended Data Fig. 7
X-ray absorption near-edge structure (XANES) characterization of SOI.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khanppnavar, B., Choo, J.P.S., Hagedoorn, PL. et al. Structural basis of the Meinwald rearrangement catalysed by styrene oxide isomerase. Nat. Chem. (2024). https://doi.org/10.1038/s41557-024-01523-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41557-024-01523-y
- Springer Nature Limited