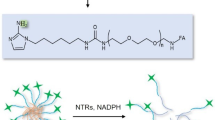
Abstract
The prognosis with pancreatic cancer is among the poorest of any human cancer. One of the important factors is the tumor hypoxia. Targeting tumor hypoxia is considered a desirable therapeutic option. However, it has not been translated into clinical success in the treatment of pancreatic cancer. With enhanced cytotoxicities against hypoxic pancreatic cancer cells, BE-43547A2 (BE) may serve as a promising template for hypoxia target strategy. Here, based on rational modification, a BE prodrug (NMP-BE) is encapsulated into sulfonated azocalix[5]arene (SAC5A) to generate a supramolecular dual hypoxia-responsive complex NMP-BE@SAC5A. Benefited from the selective load release within cancer cells, NMP-BE@SAC5A markedly suppresses tumor growth at low dose in pancreatic cancer cells xenograft murine model without develo** systemic toxicity. This research presents a strategy for the modification of covalent compounds to achieve efficient delivery within tumors, a horizon for the realization of safe and reinforced hypoxia target therapy using a simple approach.
Similar content being viewed by others
Introduction
Pancreatic cancer is a leading cause of cancer death worldwide and its global burden has increased dramatically over the past years1,2. The latest statistics show that the 5-year overall survival rate of pancreatic cancer is merely 12.5% (https://seer.cancer.gov), which is much lower compared with many other cancer types, rendering it a major medical challenge2,3. Pancreatic cancer is commonly characterized by severe hypoxia regions, it represents one of the most hypoxic cancer with < 3 mmHg pO2 in portions of tumor tissues, more than a ten-fold decrease compared to normal tissues4,5. Hypoxia contributes to the aggressiveness of pancreatic ductal adenocarcinoma (PDAC) by provoking malignant epithelial-mesenchymal transition6, enrichment of cancer stem cell population7, and strengthened glycolysis8,9,10. Besides, hypoxia predicts aggressive growth in pancreatic cancer xenografts11 and the hypoxia-inducible transcription factors, HIF-1α, is considered a predictor of clinical outcome in patients with PDAC12. Consequently, hypoxia-targeted therapeutics have emerged as promising options in the precisive treatment of pancreatic cancer13,14. Although candidates targeting hypoxia-related proteins (HIF-1α15,16,17,18 or mTOR19,20) or glycolysis21,22 proved effective in laboratory investigations or early phase clinical studies, none of them has paved its way into the market as anti-PDAC drug to the best of our knowledge13,14.
When O2 is limited, certain chemical functional groups have the potential to be metabolized by enzymatic reduction23. Bioreductively activated prodrugs converting hypoxia-sensitive cytotoxins to their active forms primarily target DNA24,25 and there is already clinical evidence for their activities against pancreatic cancer24. Nevertheless, the outcomes of clinical phase III studies on bioreductive prodrugs are disappointing26. Besides the limited extravascular penetration of prodrugs, the other key weakness is that their activation is largely dependent on the reductase activity and/or cellular reduction potential in cancer cells. However, there is individual variability among clinical patients, and the clinical efficacy of the bioreductive prodrug cannot be guaranteed24,26,27.
Thereafter, hypoxia-selective medications that work through different modes of action are eagerly warranted, not only for therapeutic purposes but for the discovery of hypoxia-related mechanisms underlying the resistance and malignant progression of tumor. However, such compounds are rare. For example, after screening 20,000 different cultivated broths of microorganisms, Ikeda et al. discovered that rakicidin A (a 4-amido-2,4-pentadieneoate (APD) cyclodepsipeptide displayed moderate hypoxia-selective cytotoxicity28. In 2017, Poulsen et al. revealed that another type of APD cyclodepsipeptide BE-43547 compounds also exhibited hypoxia-selective cytotoxicity with a significant up to 60-fold decreased IC50 value against pancreatic cancer cells (PANC1) under hypoxia compared to normoxia29. Subsequently, they made a breakthrough in demonstrating that rather than the regular DNA targeting pattern, APD cyclodepsipeptides induced fast collapse of mitochondrial function and ultrastructural integrity in hypoxic cancer cells30. More recently, we synthesized the BE alkynyl probe upon which we disclosed that BE covalently binds the cysteine234 residue of eukaryotic translation elongation factor 1 alpha 1 (eEF1A1) to exert its anti-pancreatic cancer effects31. Following this, eEF1A1 was found highly expressed under hypoxia in pancreatic cancer cells32 and acts a vital role in regulating the stemness of pancreatic cancer cells31. Besides, utilizing 99 clinical specimens of pancreatic cancer patients, eEF1A1 protein levels are found positively correlated with pancreatic cancer stage but negatively correlated with patient survival31. This highlights the importance of eEF1A1 in the progression of pancreatic cancer. Specifically targeting eEF1A1, BE may serve as a promising lead for hypoxia target therapy in pancreatic cancer treatment.
We have worked out synthetic routes that could supply BE and other structure similar natural products or their derivatives33,34,35,36,37,38,39 for in vivo experiments29,40,41,42,43,44 refers to the synthetic study of other researches. However, low water solubility and toxicity of BE limited its further application as anticancer reagent and there is a clear need to develop a functional formulation of BE to reduce its side effects and improve its anticancer effects. As a supramolecular carrier, calixarenes have been used as a molecular vessel to transport therapeutic medications into tumors, thereby enhancing therapeutic efficacy and/or alleviating side effects45,46,47. In contrast to conventional nanoscale drug-delivery systems, calixarenes feature well-defined molecular structures and weight as well as operational simplicity, which could assure batch-to-batch consistency through rigorous manufacturing procedures46,47,48,49,50. Moreover, the unusual properties of tunable cavity size and convenient modification empower these macrocyclic hosts with intriguing molecular recognition ability, thereby quantitatively binding a variety of drug guests50,51,52. In our issue, an ideal calixarene host should own the following specific characteristics: (i) high binding affinity between host and BE to prevent unwanted leaking45,53, and (ii) efficient delivery to tumors by targeting the characteristics of tumor microenvironment50,54,55,56,57. As a result, the key challenge emerges to be designing promising calixarene macrocycles with good water solubility, excellent biocompatibility, excellent binding affinity to guest molecules and targeting ability.
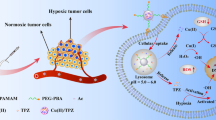
In this work, we proposed a fusion of covalent and non-covalent drug delivery strategy for the treatment of pancreatic cancer. The active and susceptible covalent binding site of BE was temporarily masked by N-methyl-piperizine via a 1,6-conjugate addition to generate an amine-adducted prodrug N-methyl-piperizine-BE-43547A2 (NMP-BE). With similar inhibitory activities against PANC-1 cells and reduced toxicity against normal cells at certain concentrations. NMP-BE is further capable of being encapsulated non-covalently into a complex with sulfonated azocalix[5]arene (SAC5A). The azo groups of azocalixarene are hypoxia-responsive50,54, and can be reduced by overexpressed azoreductases in hypoxic cancer cells. SAC5A can promote the accumulation of payload toward the tumors. When NMP-BE is unloaded within cancer cells, it could subsequently release the hypoxia-sensitive toxin BE to achieve a host-guest dual hypoxia-responsive therapeutic purpose (Fig. 1). This innovative formulation was tested in vitro and further in vivo using a PANC-1 xenograft murine model. NMP-BE@SAC5A significantly suppressed tumor growth and boosted the antitumor efficacy of NMP-BE, accompanied by no systemic toxicity observed.
The supermolecular prodrug NMP-BE@SAC5A is formed by the host-guest complexation of hypoxia-responsive molecular container SAC5A with NMP-BE. The azo groups of SAC5A quickly respond under hypoxic condition, leading to the release of loads within tumors. NMP-BE can subsequently release hypoxia-sensitive toxin BE to exert anticancer effects.
Results
Design and preparation of NMP-BE, SAC5A and NMP-BE@SAC5A
We formerly used N-methyl-piperizine (NMP) for the 1,4-conjugate addition on ovatodiolide and its derivativeSamples The pH 7.4 PBS solution was made by dissolving precise quantities of sodium phosphate monobasic dehydrate, disodium phosphate, sodium chloride, and potassium chloride in double-distilled water. The volume was then adjusted to 1000 mL with double-distilled water. The samples of NMP-BE@SAC5A were prepared by grinding. 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded on a Bruker AV400 spectrometer or a Zhongke-Niu** BIXI-I 400 spectrometer for structural characterization of compounds. Fluorescence measurements were recorded on a Cary Eclipse for the fluorescence titrations. Fluorescence microscopy images were examined using a confocal laser scanning microscope (Leica TCS SP8). Tissue sections were observed and photographed with an optical microscope (CX41, Olympus, Japan). NMP-BE was weighed and dissolved in HEPES (0.01 M) with pH of 7.4, then prepared into a solution with a concentration of 5 μg/mL. The buffer was extracted at different time points, and the peak areas of BE were detected by high performance liquid chromatography (HPLC), and the concentration of BE was calculated by external standard method. HPLC conditions: Shimadzu 20AT high performance liquid chromatography system; the mobile phase is 90% acetonitrile (0.1% H3PO4) and 10% water (0.1% H3PO4); the flow rate is 1 mL/min; the detector is UV detector; detection wavelength 210 nm for NMP-BE and 254 nm for BE; chromatographic column is C18 column. Fluorescence titrations of SAC5A, SC5A79, and SBE-β-CD were performed in 10 mM PBS, pH 7.4. The complexation of SAC5A with reporter dye (RhB) was measured by direct fluorescence titrations. A mixed solution containing known amounts of SAC5A and RhB was sequentially injected into 2.50 mL RhB solution in a quartz cuvette. The dye concentrations in mixed solution and cuvette are the same to keep dye concentration constant in the course of titrations. The fluorescence intensity was measured (λex = 554 nm for RhB) before the first addition and after every addition until a plateau was reached. By fitting the fluorescence intensity (λem = 575 nm for RhB) according to a 1:1 host-guest binding stoichiometry, the association constant was obtained. SC5A (LCG) and SBE-β-CD (NR) used the same method. The complexation of SAC5A with NMP-BE was measured by competitive fluorescence titrations. A mixed solution containing known amounts of reporter dye (RhB), host (SAC5A) and competitive guest (NMP-BE) was injected into 2.50 mL RhB and SAC5A solution in a quartz cuvette. Care was taken to keep the concentrations of dye and SAC5A constant in the course of titrations. The fluorescence intensity was measured (λex = 554 nm for RhB) before the first addition and after every addition. The association constant was obtained by fitting fluorescence intensity (λem = 575 nm for RhB) according to a 1:1 competitive binding model. The fitting of data from direct titrations and competitive titrations was performed in a nonlinear manner, and the fitting modules were downloaded from the website of Prof. Nau’s group (http://www.jacobs-university.de/ses/wnau) under the column of “Fitting Functions”. PANC1 and hTERT-HPNE cell lines were purchased from BeNa Culture Collection (Bei**g, China, BNCC352264, BNCC338221), authenticated by STR profiling and tested for mycoplasma contamination. All cells were tested for mycoplasma contamination and had no mycoplasma contamination. None of the cell lines used are classified as commonly misidentified lines. Cells were cultured in DMEM medium (Corning, Manassas, VA, USA) supplemented with 10% fetal bovine serum (FBS, Gibco Life Technologies, Grand Island, NY, USA) in a humidified incubator with 5% CO2 at 37 °C. A humidified atmosphere containing 5% CO2 was used as normoxic cell culture environment. The hypoxic cell culture environment was adjusted by purging gas mixture (94% N2, 5% CO2, 1% O2). To determine the cell viabilities of NMP, BE, and NMP-BE, PANC1 cells (5 × 103 cells/well) were seeded into the 96-well plate and incubated overnight. Then various concentrations of NMP, BE or NMP-BE were added to cells. After 72 h, 20 μL MTT solution (5 mg/mL) was added and incubated for 4 h. The OD values were determined at 570 nm by using a micro-plate reader. And the IC50 were calculated by GraphPad Prism. To determine the hypoxic selectivity of NMP-BE, PANC1 cells (5 × 103 cells/well) were seeded into the 96-well plate. After 24 h, the cells were treated with NMP-BE of different concentrations and incubated for 24 h under normoxic or hypoxic conditions, respectively. And the cell viabilities were measured by MTT assays. To determine the cell viabilities of BE, NMP-BE, and NMP-BE@SAC5A on normal cell line, HPNE cells (5 × 103 cells/well) were seeded into the 96-well plate and incubated overnight. Then various concentrations of BE, NMP-BE or NMP-BE@SAC5A were added to cells. After 24 h treatment of compounds, cell viabilities were measured by MTT assays. The cytotoxicity assays of SAC5A and SBE-β-CD and the anti-proliferation ability of NMP-BE, NMP-BE@SBE-β-CD, and NMP-BE@SAC5A were measured by cell counting kit-8 assays. PANC1 cells (5 × 103 cells/well) were seeded into the 96-well plate and incubated overnight. They were treated with SAC5A or SBE-β-CD of different concentrations and incubated for 24 h under normoxic or hypoxic conditions, respectively. In addition, the cells were treated with NMP-BE, NMP-BE@SBE-β-CD, and NMP-BE@SAC5A of different concentrations. After a 6 h incubation under normoxic conditions, the culture medium was exchanged with fresh medium. Subsequently, the cells were incubated at 37 °C for 18 h under either normoxic or hypoxic conditions, respectively. And then, the culture medium was replaced with fresh medium and 10 μL cell counting kit-8 solution (C6005, US Everbright, Jiangsu, China). Following a 4 h incubation, the optical density was assessed at 450 nm employing a microplate reader. PANC1 cells (1 × 105 cells/well) were seeded into the confocal imaging chambers. After 24 h, the cells were treated with CY5-DM, CY5-DM@SBE-β-CD, and CY5-DM@SAC5A (10/10 μM). After a 6 h incubation under normoxic conditions, the culture medium was exchanged with fresh medium. Thereafter, the cells were incubated at 37 °C for 18 h under either normoxic or hypoxic conditions, respectively. Subsequently, the cells were fixed in 4% paraformaldehyde (PFA; Biosharp, Hefei, China) for 15 min, washed with PBS buffer three times and then imaged using CLSM. Cell nuclei were counterstained with DAPI (C0060, Solarbio, Bei**g, China) for 5 min. To examine the cellular uptake mechanism of SAC5A complex, PANC1 cells (3 × 105 cells/well) were seeded into the six-well plates and incubated overnight. The cells were pretreated with different endocytosis inhibitors for 1 h: chlorpromazine (CHP, 20 μM, an inhibitor of clathrin-mediated endocytosis), Amiloride (AMI, 500 μM, an inhibitor of macropinocytosis), Genistein (GEN, 200 μM, an inhibitor of caveolae-mediated endocytosis), methyl-β-cyclodextrin (M-β-CD, 5 mM, an inhibitor of lipid rafts-mediated endocytosis), the cells were also pre-incubated under 4 °C for 1 h (energy-dependent endocytosis). Next, the cells were treated with CY5-DM@SAC5A (10/10 μM) for another 1 h. After that, the cells were collected and washed three times with PBS, and then analyzed by flow cytometry. All experiments were carried out four times80. To verify the co-localization of SAC5A and lysosome, PANC1 cells (2 × 104 cells/well) were seeded into confocal dish and incubated overnight. Then the cells were treated with CY5-DM@SAC5A (10/10 μM) for 1, 3, and 6 h, respectively. After that, cells were stained with Lyso Tracker (50 nM) for 30 min at 37 °C, and then stained with DAPI. The cells were washed with PBS buffer and imaged using CLSM. For animals and tumor model, male BALB/c nude mice at 5–6 weeks were purchased from Vital River Laboratory Animal Technology (Bei**g, China). All animal experiments were performed according to the institutional animal care guidelines established by the Institutional Animal Care and Use Committee of Nankai University. To establish the PANC1 tumor-bearing mouse model, 1 × 106 PANC1 cells were injected subcutaneously into the right chest of BALB/c nude mice. After the tumor grows to a suitable size, the tumor was divided into small pieces and then subcutaneously incubated into the right chest of other mice. When the tumor volumes of mice were around 200 mm3, the mice were randomized into three groups and intravenously injected with 100 μL of CY5-DM (200 μM), CY5-DM@SBE-β-CD (200 μM), and CY5-DM@SAC5A (200 μM). In vivo fluorescence imaging of CY5-DM, CY5-DM@SBE-β-CD, and CY5-DM@SAC5A were imaged by IVIS Lumina imaging system (Caliper Life Science, USA) at the time of 1, 3, 6, 12, and 24 h after injection, respectively. The ex vivo fluorescence imaging of major organs at 24 h post injection was imaged by IVIS. Fluorescent images were analyzed using Living Image 3.1 (Caliper Life Sciences). (Since cyanine dyes can be distributed in many organs due to their non-targeting nature, notably in the kidney. If the tumor was inoculated in the flank, the fluorescence signals of tumor might overlap with the signals of kidney and abdominal area during the living imaging. Therefore, to observe the distribution of the fluorescence signals overspread their bodies, the tumor was inoculated in the chest). For acute toxicity of NMP-BE fumaric acid, the mice were intravenously administered with different dosage of compound (0, 10, 25, 50 mg/kg) for 10 days, respectively. Body weights were measured before administration and at daily intervals after administration (n = 3). All UPLC-MS/MS analysis was carried out on an Ultra performance liquid chromatographic system (ExionLC, SCIEX, USA). The chromatographic column was Waters XSelect® HSS T3 C18 (2.1 × 50 mm, 2.5 μm). Mass spectrometric detection was performed on Triple QuadTM 4500 mass spectrometer with an electrospray ionization (ESI) interface operating in positive ion mode, was manufactured by SCIEX (Framingham, USA). The MS/MS system was operated at unit resolution in the multiple reaction monitoring (MRM) mode, and the monitored transitions were m/z 664.6 → 196.4 for NMP-BE, m/z 564.5 → 167.2 for BE. Male CD-1 mice at 6–8 weeks were purchased from Vital River Laboratory Animal Technology (Bei**g, China). Mouse were housed at 22 ± 2 °C and 55 ± 5% (relative humidity) under a 12 h light-dark cycle. Blood samples were collected at 2, 5, 15, 30, 45 min and 1, 2, 4, 8 and 24 h post-dose into heparinized tubes. Plasma was obtained after centrifugation and stored at –80 °C until they were analyzed. The plasma concentration-time profiles of NMP-BE and BE in mouse were plotted. To evaluate the tumor hypoxia at the early stage in PANC1-bearing BALB/c nude mice, immunofluorescence analysis was conducted using a HypoxyprobeTM−1 Omni Kit (HP3−100; Hypoxyprobe, Burlington, MA, USA). Pimonidazole HCl (Hypoxuprobe-1, 60 mg/kg) was injected intraperitoneally 1 h before they were killed. Staining was performed according to the manufacturer’s instructions. The tissue sections were cut and fixed in cold acetone for 10 min. The sections were rinsed and incubated overnight at 4 °C with rabbit anti-pimonidazole antibodies (PAb2627AP, 1:100). The sections were then incubated for 2 h with FITC-conjugated goat-anti-rabbit antibody (Thermo, 1:1000). Between all steps of the staining procedure, the sections were rinsed three times with for 5 min in PBS and imaged by CLSM. For in vivo anticancer efficacy study, 1 × 106 PANC1 cells were injected subcutaneously into the right flanks of the BALB/c nude mice. To investigate the anticancer effects of SBE-β-CD@NMP-BE, the mice with tumor volumes at around 50 mm3 were randomized into four groups (6 mice per group) and injected intravenously via tails with 200 μL of PBS, NMP-BE (5 mg/kg), SBE-β-CD (15 mg/kg) and NMP-BE@SBE-β-CD (20 mg/kg) every two days for 8 times and the tumor volumes for 14 days were continuously monitored. Tumor were measured by using a Vernier calipers and the volume (V) was calculated to be V = d2 × D/2, where d is the shortest and the D is longest diameter of the tumor in mm respectively. To investigate the anticancer effects of NMP-BE@SAC5A, the mice with tumor volumes at around 50 nm3 were randomized into four groups (6 mice per group) and injected intravenously via tails with 200 μL of PBS, NMP-BE (5 mg/kg), SAC5A (15 mg/kg), NMP-BE@SAC5A (20 mg/kg) every two days for 10 times and the tumor volumes for 14 days were continuously monitored. Tumors were measured as mentioned above. To assess potential toxicities, mice were monitored for weight loss. Tumors were collected for H&E analysis and immunofluorescence staining. Major organs including heart, liver, spleen, lung, and kidney, were collected and stained with H&E for histopathologic analysis. To evaluate biosafety of SAC5A, blood samples were collected for blood chemistry assay and blood routine assay. All results are presented as the mean ± standard deviation (SD) as indicated. Data were analyzed by one- or two-way analysis of variance (ANOVA) of comparison of multiple groups using the GraphPad Prism. P values less than 0.05 were considered statistically significant. Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.Apparatus
In vitro release rate of BE-43547A2 (BE) from prodrug NMP-BE
Data analyses of fluorescence titrations
Cell culture
In vitro cytotoxicity assays
Confocal laser scanning microscopy (CLSM)
Cellular uptake of SAC5A
In vivo fluorescence imaging
Acute toxicity of NMP-BE fumaric acid
Experimental procedure of the pharmacokinetic (PK) study of NMP-BE
Detection of tumor hypoxia
In vivo anticancer efficacy study
Statistical analysis
Reporting summary
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information, and from the corresponding author upon request. The source data underlying Figs. 2c–e, 3d–f, 4b, c, 5b, d, e, 6a–l and Supplementary Figs. 7, 8, 9, 10, 11, 12, 14, 15a, b, 16, 19a, b, 20, 22a, c, and 23a, c, d are provided as a Source Data file. The full image dataset is available from the corresponding author upon request. Source data are provided with this paper.
References
Klein, A. P. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat. Rev. Gastroenterol. Hepatol. 18, 493–502 (2021).
Chen, X., Zeh, H. J., Kang, R., Kroemer, G. & Tang, D. Cell death in pancreatic cancer: from pathogenesis to therapy. Nat. Rev. Gastroenterol. Hepatol. 18, 804–823 (2021).
Wood, L. D., Canto, M. I., Jaffee, E. M. & Simeone, D. M. Pancreatic Cancer: pathogenesis, screening, diagnosis, and treatment. Gastroenterology 163, 386–402.e381 (2022).
Koong, A. C. et al. Pancreatic tumors show high levels of hypoxia. Int. J. Radiat. Oncol. 48, 919–922 (2000).
Vaupel, P., Hockel, M. & Mayer, A. Detection and characterization of tumor hypoxia using pO2 histography. Antioxid. Redox Sign. 9, 1221–1235 (2007).
Salnikov, A. V. et al. Hypoxia induces EMT in low and highly aggressive pancreatic tumor cells but only cells with cancer stem cell characteristics acquire pronounced migratory potential. PLoS ONE 7, e46391 (2012).
Hashimoto, O. et al. Hypoxia induces tumor aggressiveness and the expansion of CD133-positive cells in a hypoxia-inducible factor-1alpha-dependent manner in pancreatic cancer cells. Pathobiology 78, 181–192 (2011).
Guillaumond, F. et al. Strengthened glycolysis under hypoxia supports tumor symbiosis and hexosamine biosynthesis in pancreatic adenocarcinoma. Proc. Natl. Acad. Sci. USA 110, 3919–3924 (2013).
Halbrook, C. J. & Lyssiotis, C. A. Employing metabolism to improve the diagnosis and treatment of pancreatic cancer. Cancer Cell 31, 5–19 (2017).
Qin, Y. et al. LSD1 sustains pancreatic cancer growth via maintaining HIF1alpha-dependent glycolytic process. Cancer Lett. 347, 225–232 (2014).
Chang, Q., Jurisica, I., Do, T. & Hedley, D. W. Hypoxia predicts aggressive growth and spontaneous metastasis formation from orthotopically grown primary xenografts of human pancreatic cancer. Cancer Res. 71, 3110–3120 (2011).
Hoffmann, A. C. et al. High expression of HIF1a is a predictor of clinical outcome in patients with pancreatic ductal adenocarcinomas and correlated to PDGFA, VEGF, and bFGF. Neoplasia 10, 674–679 (2008).
Erkan, M., Kurtoglu, M. & Kleeff, J. The role of hypoxia in pancreatic cancer: a potential therapeutic target? Expert Rev. Gastroent. 10, 301–316 (2016).
Tao, J. et al. Targeting hypoxic tumor microenvironment in pancreatic cancer. J. Hematol. Oncol. 14, 14 (2021).
Semenza, G. L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 3, 721–732 (2003).
**, X., Dai, L., Ma, Y., Wang, J. & Liu, Z. Implications of HIF-1alpha in the tumorigenesis and progression of pancreatic cancer. Cancer Cell Int. 20, 273 (2020).
Zhao, T. et al. Inhibition of HIF-1alpha by PX-478 enhances the anti-tumor effect of gemcitabine by inducing immunogenic cell death in pancreatic ductal adenocarcinoma. Oncotarget 6, 2250–2262 (2015).
Jeong, W. et al. Pilot trial of EZN-2968, an antisense oligonucleotide inhibitor of hypoxia-inducible factor-1 alpha (HIF-1alpha), in patients with refractory solid tumors. Cancer Chemoth. Pharm. 73, 343–348 (2014).
Wolpin, B. M. et al. Oral mTOR inhibitor everolimus in patients with gemcitabine-refractory metastatic pancreatic cancer. J. Clin. Oncol. 27, 193–198 (2009).
Javle, M. M. et al. Inhibition of the mammalian target of rapamycin (mTOR) in advanced pancreatic cancer: results of two phase II studies. BMC Cancer 10, 368 (2010).
Raez, L. E. et al. A phase I doseescalation trial of 2-deoxy-D-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemoth. Pharm. 71, 523–530 (2013).
Lampidis, T. J. et al. Efficacy of 2-halogen substituted D-glucose analogs in blocking glycolysis and killing “hypoxic tumor cells”. Cancer Chemoth. Pharm. 58, 725–734 (2006).
Wilson, W. R. & Hay, M. P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 11, 393–410 (2011).
Hunter, F. W., Wouters, B. G. & Wilson, W. R. Hypoxia-activated prodrugs: paths forward in the era of personalised medicine. Br. J. Cancer 114, 1071–1077 (2016).
Guise, C. P. et al. Bioreductive prodrugs as cancer therapeutics: targeting tumor hypoxia. Chin. J. Cancer 33, 80–86 (2014).
Spiegelberg, L. et al. Hypoxia-activated prodrugs and (lack of) clinical progress: the need for hypoxia-based biomarker patient selection in phase III clinical trials. Clin. Transl. Rad. Oncol. 15, 62–69 (2019).
Dhani, N. C. et al. Analysis of the intra- and intertumoral heterogeneity of hypoxia in pancreatic cancer patients receiving the nitroimidazole tracer pimonidazole. Br. J. Cancer 113, 864–871 (2015).
Yamazaki, Y., Kunimoto, S. & Ikeda, D. Rakicidin A: a hypoxia-selective cytotoxin. Biol. Pharm. Bull. 30, 261–265 (2007).
Villadsen, N. L. et al. Synthesis of ent-BE-43547A1 reveals a potent hypoxia-selective anticancer agent and uncovers the biosynthetic origin of the APD-CLD natural products. Nat. Chem. 9, 264–272 (2017).
Jacobsen, K. M. et al. APD-containing cyclolipodepsipeptides target mitochondrial function in hypoxic cancer cells. Cell Chem. Biol. 25, 1337–1349.e12 (2018).
Liu, C. et al. Probe synthesis reveals eukaryotic translation elongation factor 1 alpha 1 as the anti-pancreatic cancer target of BE-43547A2. Angew. Chem. Int. Ed. 61, e202206953 (2022).
Liu, C. et al. BE-43547A2 achieves hypoxia selectivity by targeting eEF1A1 and disrupting its association with FoxO1 in pancreatic cancer. Cell Chem. Biol. CELL-CHEMICAL-BIOLOGY-D-22-00554 (manuscript in revision).
Sun, Y. et al. Cyclic depsipeptide BE-43547A2: synthesis and activity against pancreatic cancer stem cells. Angew. Chem. Int. Ed. 56, 14627–14631 (2017).
Sun, Y. et al. Syntheses and biological evaluation of BE-43547A2 analogues modified at O35 ester and C15-OH sites. Tetrahedron 75, 1808–1818 (2019).
Sun, Y. et al. Total synthesis of BE-43547A2. Tetrahedron 74, 5955–5964 (2018).
Sang, F. et al. Total synthesis and determination of the absolute configuration of rakicidin A. J. Am. Chem. Soc. 136, 15787–15791 (2014).
Sang, F. et al. Structure-activity relationship study of rakicidins: overcoming chronic myeloid leukemia resistance to imatinib with 4-methylester-rakicidin A. J. Med. Chem. 59, 1184–1196 (2016).
Wang, J. et al. Total syntheses and biological activities of vinylamycin analogues. J. Med. Chem. 60, 1189–1209 (2017).
Chen, J. et al. Syntheses and anti-pancreatic cancer activities of rakicidin analogues. Eur. J. Med. Chem. 151, 601–627 (2018).
Tsakos, M. et al. Total synthesis and biological evaluation of rakicidin A and discovery of a simplified bioactive analogue. Angew. Chem. Int. Ed. 55, 1030–1035 (2016).
Poulsen, T. B. A concise route to the macrocyclic core of the rakicidins. Chem. Commun. 47, 12837–12839 (2011).
Clement, L. L. et al. The amido-pentadienoate-functionality of the rakicidins is a thiol reactive electrophile–development of a general synthetic strategy. Chem. Commun. 51, 12427–12430 (2015).
Tsakos, M., Jacobsen, K., Yu, W., Villadsen, N. L. & Poulsen, T. B. The rakicidin family of anticancer natural products-synthetic strategies towards a new class of hypoxia-selective cytotoxins. Synlett 27, 1898–1906 (2016).
Kranthikumar, R. Toward the synthesis of the hypoxia selective anticancer agent BE-43547A2. Org. Biomol. Chem. 19, 9833–9839 (2021).
Webber, M. J. & Langer, R. Drug delivery by supramolecular design. Chem. Soc. Rev. 46, 6600–6620 (2017).
Pan, Y.-C., Hu, X.-Y. & Guo, D.-S. Biomedical applications of calixarenes: state of the art and perspectives. Angew. Chem. Int. Ed. 60, 2768–2794 (2021).
Pan, Y.-C. et al. Coassembly of macrocyclic amphiphiles for anti-β-amyloid therapy of alzheimer’s disease. CCS Chem. 3, 2485–2497 (2021).
Zheng, Z. et al. Guanidinocalix[5]arene for sensitive fluorescence detection and magnetic removal of perfluorinated pollutants. Nat. Commun. 10, 5762 (2019).
Zhang, Z. et al. Macrocyclic-amphiphile-based self-assembled nanoparticles for ratiometric delivery of therapeutic combinations to tumors. Adv. Mater. 33, e2007719 (2021).
Zhang, T.-X. et al. A general hypoxia-responsive molecular container for tumor-targeted therapy. Adv. Mater. 32, e1908435 (2020).
Zhang, T.-X. et al. A hypoxia-responsive supramolecular formulation for imaging-guided photothermal therapy. Theranostics 12, 396–409 (2022).
Tian, J.-H. et al. A facile way to construct sensor array library via supramolecular chemistry for discriminating complex systems. Nat. Commun. 13, 4293 (2022).
Yu, G. & Chen, X. Host-guest chemistry in supramolecular theranostics. Theranostics 9, 3041–3074 (2019).
Geng, W.-C. et al. A noncovalent fluorescence turn-on strategy for hypoxia imaging. Angew. Chem. Int. Ed. 58, 2377–2381 (2019).
Cheng, Y.-Q. et al. Coassembly of hypoxia-sensitive macrocyclic amphiphiles and extracellular vesicles for targeted kidney injury imaging and therapy. J. Nanobiotechnol. 19, 451 (2021).
Hou, X. et al. Supramolecular radiosensitizer based on hypoxia-responsive macrocycle. Adv. Sci. 9, e2104349 (2022).
Wang, H. et al. Self-motivated supramolecular combination chemotherapy for overcoming drug resistance based on acid-activated competition of host–guest interactions. CCS Chem. 3, 1413–1425 (2021).
**ang, J. et al. Chemical modification of ovatodiolide revealed a promising amino-prodrug with improved pharmacokinetic profile. Chem. Commun. 56, 11018–11021 (2020).
Zhang, Q. et al. Guaianolide sesquiterpene lactones, a source to discover agents that selectively inhibit acute myelogenous leukemia stem and progenitor cells. J. Med. Chem. 55, 8757–8769 (2012).
Morgenthaler, M. et al. Predicting and tuning physicochemical properties in lead optimization: amine basicities. ChemMedChem 2, 1100–1115 (2007).
Zhang, T.-X., Li, J.-J., Li, H.-B. & Guo, D.-S. Deep cavitand calixarene-solubilized fullerene as a potential photodynamic agent. Front. Chem. 9, 710808 (2021).
Yue, Y.-X. et al. Promoting tumor accumulation of anticancer drugs by hierarchical carrying of exogenous and endogenous vehicles. Small Struct. 2200067 (2022).
Mironova, D. et al. Azocalix[4]arene-rhodamine supramolecular hypoxia-sensitive systems: a search for the best calixarene hosts and rhodamine guests. Molecules 26, 5451 (2021).
Galieva, F. et al. New supramolecular hypoxia-sensitive complexes based on azo-thiacalixarene. Molecules 28, 466 (2023).
Xu, P. et al. Targeted charge-reversal nanoparticles for nuclear drug delivery. Angew. Chem. Int. Ed. 46, 4999–5002 (2007).
Wong, P. T. & Choi, S. K. Mechanisms of drug release in nanotherapeutic delivery systems. Chem. Rev. 115, 3388–3432 (2015).
Sun, Q., Zhou, Z., Qiu, N. & Shen, Y. Rational design of cancer nanomedicine: nanoproperty integration and synchronization. Adv. Mater. 29, 1606628 (2017).
Wang, S. et al. Study on the optical and biological properties in vitro of IR808-PEG-FA. J. Biomed. Mater. Res. A 108, 1816–1823 (2020).
Kang, H. et al. Renal clearable theranostic nanoplatforms for gastrointestinal stromal tumors. Adv. Mater. 32, e1905899 (2020).
Bushweller, J. H. Targeting transcription factors in cancer - from undruggable to reality. Nat. Rev. Cancer 19, 611–624 (2019).
Chen, S. L. et al. eEF1A1 overexpression enhances tumor progression and indicates poor prognosis in hepatocellular carcinoma. Transl. Oncol. 11, 125–131 (2018).
Liu, S. et al. METTL13 methylation of eEF1A increases translational output to promote tumorigenesis. Cell 176, 491–504 (2019).
Leisch, M., Egle, A. & Greil, R. Plitidepsin: a potential new treatment for relapsed/refractory multiple myeloma. Future Oncol. 15, 109–120 (2019).
Gomes, N. G. M., Valentao, P., Anrade, P. B. & Pereira, R. B. Plitidepsin to treat multiple myeloma. Drug. Today 56, 337–347 (2020).
Janoniene, A. et al. A versatile carbonic anhydrase IX targeting ligand-functionalized porous silicon nanoplatform for dual hypoxia cancer therapy and imaging. ACS Appl. Mater. Interfaces 9, 13976–13987 (2017).
Zhu, R. et al. Cancer-selective bioreductive chemotherapy mediated by dual hypoxia-responsive nanomedicine upon photodynamic therapy-induced hypoxia aggravation. Biomacromolecules 20, 2649–2656 (2019).
Ihsanullah, K. M. et al. Stepwise-activatable hypoxia triggered nanocarrier-based photodynamic therapy for effective synergistic bioreductive chemotherapy. Biomaterials 245, 119982 (2020).
Li, Y. et al. Dual hypoxia-targeting RNAi nanomedicine for precision cancer therapy. Nano Lett. 20, 4857–4863 (2020).
Liu, Y.-C., Wang, Y.-Y., Tian, H.-W., Liu, Y. & Guo, D.-S. Fluorescent nanoassemblies between tetraphenylethenes and sulfonatocalixarenes: a systematic study of calixarene-induced aggregation. Org. Chem. Front. 3, 53–61 (2016).
Tuo, Wei et al. Systemic nanoparticle delivery of CRISPR-Cas9 ribonucleoproteins for effective tissue specific genome editing. Nat. Commun. 11, 3232 (2020).
Acknowledgements
Financial support from NSFC (U20A20259 and 31961143004 to D.-S.G.), the Fundamental Research Funds for the Central Universities, the NCC Fund (Grant No. NCC2020FH04 to D.-S.G.), the Haihe Laboratory of Sustainable Chemical Transformations, and the Postdoctoral Science Foundation of China (2021M701791) are gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
J.S.G. and J.J.L. contributed equally. D.S.G., L.W., and Y.C. devised the project. J.S.G. and J.J.L. carried out the experimental work and analyzed the data. Z.H.W., H.B.L., X.H.Z., and Y.J.S. synthesized the compounds. Y.X.Y., Y.L., and Y.H.D. carried out the experimental work. F.D. provided technical support for nuclear overhauser effect spectroscopy. J.S.G., J.J.L., D.S.G., and L.W. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Vladimir Burilov, Agnieszka Sliwinska and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, JS., Li, JJ., Wang, ZH. et al. Dual hypoxia-responsive supramolecular complex for cancer target therapy. Nat Commun 14, 5634 (2023). https://doi.org/10.1038/s41467-023-41388-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-41388-2
- Springer Nature Limited