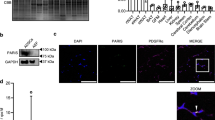
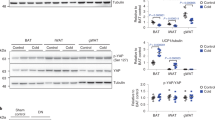
Abstract
Parkin, an E3 ubiquitin ligase, plays an essential role in mitochondrial quality control. However, the mechanisms by which Parkin connects mitochondrial homeostasis with cellular metabolism in adipose tissue remain unclear. Here, we demonstrate that Park2 gene (encodes Parkin) deletion specifically from adipose tissue protects mice against high-fat diet and aging-induced obesity. Despite a mild reduction in mitophagy, mitochondrial DNA content and mitochondrial function are increased in Park2 deficient white adipocytes. Moreover, Park2 gene deletion elevates mitochondrial biogenesis by increasing Pgc1α protein stability through mitochondrial superoxide-activated NAD(P)H quinone dehydrogenase 1 (Nqo1). Both in vitro and in vivo studies show that Nqo1 overexpression elevates Pgc1α protein level and mitochondrial DNA content and enhances mitochondrial activity in mouse and human adipocytes. Taken together, our findings indicate that Parkin regulates mitochondrial homeostasis by balancing mitophagy and Pgc1α-mediated mitochondrial biogenesis in white adipocytes, suggesting a potential therapeutic target in adipocytes to combat obesity and obesity-associated disorders.
Similar content being viewed by others
Introduction
Mitochondrial dysfunction contributes to the pathogenesis of metabolic disorders such as obesity1. Mitochondria are highly dynamic organelles that play essential roles in energy metabolism, heat production, generation of oxygen radicals, calcium signaling, and apoptosis2,3,4,5,6. To maintain metabolic health, mitochondrial selective autophagy (mitophagy), mitochondrial biogenesis, and various mitochondrial protein degradation processes coordinately control the quality of mitochondria6,7.
Mitophagy involves the recognition of damaged mitochondria by the autophagosome through microtubule-associated protein 1 light chain 3 (LC3) adapters in ubiquitin-dependent and independent mechanisms8,9. PTEN-induced putative kinase 1 (Pink1, encoded by Park6) and E3 ubiquitin-protein ligase Parkin are known to function in ubiquitin-dependent mitophagy, while other proteins such as TBC1 domain family member 15 (Tbc1d15) and Nip3-like protein X (Nix) contribute to ubiquitin-independent mitophagy9,10,11,12.
Emerging evidence has shown that low mitochondrial content and activity contribute to adipocyte hypertrophy and metabolic disorders13,14,15,S1 for a list of the primers used.
RNA sequencing and analysis
A homogenous portion of frozen adipose samples were homogenized using a tissue homogenizer in Trizol. RNA was isolated using the Qiagen RNeasy Mini QIAcube Kit following the manufacturer’s instructions. Isolated RNA was checked for concentration using a NanoDrop and purity using the Agilent TapeStation. Only samples with RIN > 7.0 were used. Libraries were prepared using the KAPA mRNA HyperPrep Kit following the manufacturer’s instructions. The resulting libraries were combined into two pools and sequenced on an Illumina HiSeq 3000 within the UCLA TCGB core facility following in-house established protocols. Raw reads were checked for quality using FastQC, aligned to the Mus musculus GRCm38, and then counted using Mus musculus GRCm38 version 97. Alignment and counting occurred using Rsubread v 2.4.263. Raw counts were then analyzed for differential gene expression using the DESeq2 v1.30.064.
Statistics
Values presented are expressed as means ± SEM unless otherwise indicated. Statistical analyses were performed using Student’s t test when comparing two groups of samples or Two-way analysis of variance (ANOVA) for identification of significance within and between groups using GraphPad Prism9.4.1 (GraphPad Software). Significance was set a priori at P < 0.05. For RNA Sequencing analysis, the significance was set as FDR < 0.05.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Source data are provided as a Source Data file. METSIM adipose array data are available from in the Gene Expression Omnibus database under accession code GSE70353, and additional clinical data were obtained from previously published papers60,65. The mouse RNA-seq results from eWAT of HFD-fed ParkinAdi vs. Control f/f mice and AAV8-Nqo1 vs. AAV8-Control injected eWAT have been deposited in the Gene Expression Omnibus database under accession code GSE207496. Human PARK2 gene (encodes Parkin protein) association is available from PhenoScanner v2 (http://www.phenoscanner.medschl.cam.ac.uk). The authors declare that all data supporting the findings of this study are available within the paper and its Supplementary Information files.
References
Bournat, J. C. & Brown, C. W. Mitochondrial dysfunction in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 17, 446–452 (2010).
McBride, H. M., Neuspiel, M. & Wasiak, S. Mitochondria: more than just a powerhouse. Curr. Biol. 16, R551–R560 (2006).
Nunnari, J. & Suomalainen, A. Mitochondria: in sickness and in health. Cell 148, 1145–1159 (2012).
Bray, N. Many makes of mitochondria. Nat. Rev. Neurosci. 20, 645 (2019).
Detmer, S. A. & Chan, D. C. Functions and dysfunctions of mitochondrial dynamics. Nat. Rev. Mol. Cell Biol. 8, 870–879 (2007).
Palikaras, K., Lionaki, E. & Tavernarakis, N. Balancing mitochondrial biogenesis and mitophagy to maintain energy metabolism homeostasis. Cell Death Differ. 22, 1399–1401 (2015).
Ni, H. M., Williams, J. A. & Ding, W. X. Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 4, 6–13 (2015).
Altshuler-Keylin, S. & Kajimura, S. Mitochondrial homeostasis in adipose tissue remodeling. Sci Signal 10, https://doi.org/10.1126/scisignal.aai9248 (2017).
Yoo, S. M. & Jung, Y. K. A molecular approach to mitophagy and mitochondrial dynamics. Mol. Cells 41, 18–26 (2018).
Yamano, K., Fogel, A. I., Wang, C., van der Bliek, A. M. & Youle, R. J. Mitochondrial Rab GAPs govern autophagosome biogenesis during mitophagy. Elife 3, e01612 (2014).
Hanna, R. A. et al. Microtubule-associated protein 1 light chain 3 (LC3) interacts with Bnip3 protein to selectively remove endoplasmic reticulum and mitochondria via autophagy. J. Biol. Chem. 287, 19094–19104 (2012).
Novak, I. et al. Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 11, 45–51 (2010).
Diaz-Vegas, A. et al. Is Mitochondrial Dysfunction a Common Root of Noncommunicable Chronic Diseases? Endocr. Rev. 41, https://doi.org/10.1210/endrev/bnaa005 (2020).
Wilson-Fritch, L. et al. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J. Clin. Investig. 114, 1281–1289 (2004).
Heinonen, S. et al. Impaired mitochondrial biogenesis in adipose tissue in acquired obesity. Diabetes 64, 3135–3145 (2015).
Bogacka, I., **e, H., Bray, G. A. & Smith, S. R. Pioglitazone induces mitochondrial biogenesis in human subcutaneous adipose tissue in vivo. Diabetes 54, 1392–1399 (2005).
Rong, J. X. et al. Adipose mitochondrial biogenesis is suppressed in db/db and high-fat diet-fed mice and improved by rosiglitazone. Diabetes 56, 1751–1760 (2007).
Ruegsegger, G. N., Creo, A. L., Cortes, T. M., Dasari, S. & Nair, K. S. Altered mitochondrial function in insulin-deficient and insulin-resistant states. J. Clin. Investig. 128, 3671–3681 (2018).
Cui, C. et al. PINK1-Parkin alleviates metabolic stress induced by obesity in adipose tissue and in 3T3-L1 preadipocytes. Biochem. Biophys. Res. Commun. 498, 445–452 (2018).
Taylor, D. & Gottlieb, R. A. Parkin-mediated mitophagy is downregulated in browning of white adipose tissue. Obes. (Silver Spring) 25, 704–712 (2017).
Lu, X. et al. Mitophagy controls beige adipocyte maintenance through a Parkin-dependent and UCP1-independent mechanism. Sci. Signal 11, https://doi.org/10.1126/scisignal.aap8526 (2018).
Cairo, M. et al. Parkin controls brown adipose tissue plasticity in response to adaptive thermogenesis. EMBO Rep. 20, https://doi.org/10.15252/embr.201846832 (2019).
Corsa, C. A. S. et al. The E3 ubiquitin ligase parkin is dispensable for metabolic homeostasis in murine pancreatic beta cells and adipocytes. J. Biol. Chem. 294, 7296–7307 (2019).
Staley, J. R. et al. PhenoScanner: a database of human genotype-phenotype associations. Bioinformatics 32, 3207–3209 (2016).
Kamat, M. A. et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics 35, 4851–4853 (2019).
**ao, D., Powolny, A. A. & Singh, S. V. Benzyl isothiocyanate targets mitochondrial respiratory chain to trigger reactive oxygen species-dependent apoptosis in human breast cancer cells. J. Biol. Chem. 283, 30151–30163 (2008).
Aquilano, K., Lettieri Barbato, D. & Rosa, C. M. The multifaceted role of nitric oxide synthases in mitochondrial biogenesis and cell differentiation. Commun. Integr. Biol. 8, e1017158 (2015).
Onishi, M., Yamano, K., Sato, M., Matsuda, N. & Okamoto, K. Molecular mechanisms and physiological functions of mitophagy. EMBO J. 40, e104705 (2021).
Kitada, M. & Koya, D. Autophagy in metabolic disease and ageing. Nat. Rev. Endocrinol. 17, 647–661 (2021).
Lu, Y. et al. Mitophagy is required for brown adipose tissue mitochondrial homeostasis during cold challenge. Sci. Rep. 8, 8251 (2018).
Yau, W. W. et al. Chronic cold exposure induces autophagy to promote fatty acid oxidation, mitochondrial turnover, and thermogenesis in brown adipose tissue. iScience 24, 102434 (2021).
Zhou, Z. et al. Estrogen receptor alpha controls metabolism in white and brown adipocytes by regulating Polg1 and mitochondrial remodeling. Sci. Transl. Med. 12, https://doi.org/10.1126/scitranslmed.aax8096 (2020).
Livingston, M. J. et al. Clearance of damaged mitochondria via mitophagy is important to the protective effect of ischemic preconditioning in kidneys. Autophagy 15, 2142–2162 (2019).
McWilliams, T. G. et al. mito-QC illuminates mitophagy and mitochondrial architecture in vivo. J. Cell Biol. 214, 333–345 (2016).
Adamovich, Y. et al. The protein level of PGC-1alpha, a key metabolic regulator, is controlled by NADH-NQO1. Mol. Cell Biol. 33, 2603–2613 (2013).
Kasai, S., Shimizu, S., Tatara, Y., Mimura, J. & Itoh, K. Regulation of Nrf2 by mitochondrial reactive oxygen species in physiology and pathology. Biomolecules 10, https://doi.org/10.3390/biom10020320 (2020).
Ross, D. & Siegel, D. Functions of NQO1 in cellular protection and CoQ10 metabolism and its potential role as a redox sensitive molecular switch. Front. Physiol. 8, 595 (2017).
Di Francesco, A. et al. NQO1 protects obese mice through improvements in glucose and lipid metabolism. NPJ Aging Mech. Dis. 6, 13 (2020).
Zucker, S. N. et al. Nrf2 amplifies oxidative stress via induction of Klf9. Mol. Cell 53, 916–928 (2014).
Palming, J. et al. The expression of NAD(P)H:quinone oxidoreductase 1 is high in human adipose tissue, reduced by weight loss, and correlates with adiposity, insulin sensitivity, and markers of liver dysfunction. J. Clin. Endocrinol. Metab. 92, 2346–2352 (2007).
Shi, X. et al. Paradoxical effect of mitochondrial respiratory chain impairment on insulin signaling and glucose transport in adipose cells. J. Biol. Chem. 283, 30658–30667 (2008).
Wrighton, K. H. Metabolism: Mitophagy turns beige adipocytes white. Nat. Rev. Mol. Cell Biol. 17, 607 (2016).
Puigserver, P. et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839 (1998).
Wu, Z. et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98, 115–124 (1999).
Virbasius, J. V. & Scarpulla, R. C. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc. Natl Acad. Sci. USA 91, 1309–1313 (1994).
Komen, J. C. & Thorburn, D. R. Turn up the power - pharmacological activation of mitochondrial biogenesis in mouse models. Br. J. Pharm. 171, 1818–1836 (2014).
Svensson, K. & Handschin, C. Modulation of PGC-1alpha activity as a treatment for metabolic and muscle-related diseases. Drug Disco. Today 19, 1024–1029 (2014).
Popov, L. D. Mitochondrial biogenesis: An update. J. Cell Mol. Med. 24, 4892–4899 (2020).
Chella Krishnan, K. et al. Sex-specific genetic regulation of adipose mitochondria and metabolic syndrome by Ndufv2. Nat. Metab. 3, 1552–1568 (2021).
Liu, L. et al. Mitophagy receptor FUNDC1 is regulated by PGC-1alpha/NRF1 to fine tune mitochondrial homeostasis. EMBO Rep. 22, e50629 (2021).
Hwang, J. H. et al. Pharmacological stimulation of NADH oxidation ameliorates obesity and related phenotypes in mice. Diabetes 58, 965–974 (2009).
Houtkooper, R. H., Canto, C., Wanders, R. J. & Auwerx, J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr. Rev. 31, 194–223 (2010).
Kim, Y. H. et al. Activation of NAD(P)H:quinone oxidoreductase ameliorates spontaneous hypertension in an animal model via modulation of eNOS activity. Cardiovasc. Res. 91, 519–527 (2011).
Lee, J. S. et al. Beta-lapachone, a modulator of NAD metabolism, prevents health declines in aged mice. PLoS ONE 7, e47122 (2012).
Choi, W. H., Ahn, J., Jung, C. H., Jang, Y. J. & Ha, T. Y. β-lapachone prevents diet-induced obesity by increasing energy expenditure and stimulating the browning of white adipose tissue via downregulation of miR-382 expression. Diabetes 65, 2490–2501 (2016).
Shin, J. H. et al. PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson’s disease. Cell 144, 689–702 (2011).
Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 194, 7–15 (2011).
Den Hartigh, L. J. et al. Adipocyte-specific deficiency of NADPH oxidase 4 delays the onset of insulin resistance and attenuates adipose tissue inflammation in obesity. Arterioscler. Thromb. Vasc. Biol. 37, 466–475 (2017).
Issa, N. et al. Cytokines promote lipolysis in 3T3-L1 adipocytes through induction of NADPH oxidase 3 expression and superoxide production. J. Lipid Res. 59, 2321–2328 (2018).
Vangipurapu, J. et al. Association of indices of liver and adipocyte insulin resistance with 19 confirmed susceptibility loci for type 2 diabetes in 6,733 non-diabetic Finnish men. Diabetologia 54, 563–571 (2011).
Rajbhandari, P. et al. IL-10 signaling remodels adipose chromatin architecture to limit thermogenesis and energy expenditure. Cell 172, 218–233 e217 (2018).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Liao, Y., Smyth, G. K. & Shi, W. The R package Rsubread is easier, faster, cheaper and better for alignment and quantification of RNA sequencing reads. Nucleic Acids Res. 47, e47 (2019).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Orozco, L. D. et al. Epigenome-wide association in adipose tissue from the METSIM cohort. Hum. Mol. Genet. 27, 1830–1846 (2018).
Acknowledgements
We would like to thank Dr. Ted M. Dawson at Johns Hopkins University for providing us Parkin flox mice. We thank undergraduate students Julia Zhou at the University of California San Diego for analyzing the adipocyte size. We thank Wenjuan Ren for immunoblotting and Calvin Pan for HMDP data analysis. Z.Z. was supported by an NIH grant (DK125354). A.L.H. was supported by NIH grants (DK109724, P30DK063491). A.J.L. was supported by NIH grants (U54 DK120342 and DK117850). T.M.M. was supported by the UCLA Intercampus Medical Genetics Training Program (T32GM008243), NRSA predoctoral fellowship (F31DK108657), Carl V. Gisolfi Memorial Research grant from the American College of Sports Medicine, and a predoctoral graduate student award from the Dornsife College at the University of Southern California. D.M.W. is supported by an EMBO long-term fellowship ALTF 828-2021.
Author information
Authors and Affiliations
Contributions
Z.Z., T.M.M., and A.L.H conceived and designed the experiments. Z.Z., T.M.M., A.R.S., and X.Z. performed all in vivo studies. Z.Z., T.M.M., X.Z., A.R.S., Y.C., A.M., and P.S. conducted the sample collection and subsequent experimental analysis. B.L.C., and T.Q.d.A.V. performed indirect calorimetry studies. Z.Z. and T.M.M. performed all adipocyte culture studies and mtDNA content analysis. L.C. performed all AAV injection studies. D.M.W., J.N., M.S. conducted studies related to mitochondrial respirometry and confocal microscopy. Z.Z., T.M.M., L.C., X.Z., A.R.S., Y.C., B.L.C., A.M., P.S. T.Q.d.A.V., M.L., O.S.S., A.L.H, and A.J.L. analyzed the data. T.M.M., L.C., A.L.H, A.J.L., and Z.Z. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Qiong Wang and the other, anonymous, reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moore, T.M., Cheng, L., Wolf, D.M. et al. Parkin regulates adiposity by coordinating mitophagy with mitochondrial biogenesis in white adipocytes. Nat Commun 13, 6661 (2022). https://doi.org/10.1038/s41467-022-34468-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-34468-2
- Springer Nature Limited
This article is cited by
-
Mitochondrial quality control in human health and disease
Military Medical Research (2024)
-
Parkin depletion prevents the age-related alterations in the FGF21 system and the decline in white adipose tissue thermogenic function in mice
Journal of Physiology and Biochemistry (2024)
-
Mitochondrial homeostasis: sha** health and disease
Current Medicine (2024)
-
Crosstalk between mitochondrial biogenesis and mitophagy to maintain mitochondrial homeostasis
Journal of Biomedical Science (2023)
-
White adipose tissue mitochondrial bioenergetics in metabolic diseases
Reviews in Endocrine and Metabolic Disorders (2023)