Abstract
Neutrophil extracellular traps (NETs) can capture and kill viruses, such as influenza viruses, human immunodeficiency virus (HIV), and respiratory syncytial virus (RSV), thus contributing to host defense. Contrary to our expectation, we show here that the histones released by NETosis enhance the infectivity of SARS-CoV-2, as found by using live SARS-CoV-2 and two pseudovirus systems as well as a mouse model. The histone H3 or H4 selectively binds to subunit 2 of the spike (S) protein, as shown by a biochemical binding assay, surface plasmon resonance and binding energy calculation as well as the construction of a mutant S protein by replacing four acidic amino acids. Sialic acid on the host cell surface is the key molecule to which histones bridge subunit 2 of the S protein. Moreover, histones enhance cell–cell fusion. Finally, treatment with an inhibitor of NETosis, histone H3 or H4, or sialic acid notably affected the levels of sgRNA copies and the number of apoptotic cells in a mouse model. These findings suggest that SARS-CoV-2 could hijack histones from neutrophil NETosis to promote its host cell attachment and entry process and may be important in exploring pathogenesis and possible strategies to develop new effective therapies for COVID-19.
Similar content being viewed by others
Introduction
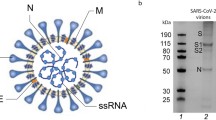
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes an infectious viral respiratory disease known as COVID-19, severely affecting global public health year-round. SARS-CoV-2 is an enveloped virus with a single-stranded positive (+)-sense RNA genome of ∼30 kb. The four structural proteins of SARS-CoV-2 are the spike (S), membrane (M), envelope (E), and nucleocapsid (N) proteins [1]. The S protein of SARS-CoV-2 consists of two subunits, S1 and S2, which are responsible for receptor recognition and the cell membrane fusion process, respectively [2]. SARS-CoV-2 first binds to the ACE2 receptor on the host cell surface via the virion RBD in S1. Viral attachment and host protease cleavage facilitate conformational changes in the S protein and the insertion of the fusion peptide of the S protein into the target membrane. Subsequently, the heptad repeat 1 (HR1) and 2 (HR2) domains in S2 interact with each other to form a stable six-helix bundle fusion core and eventually accelerate the viral and cellular membrane fusion process [Binding of the spike protein to histones The flat-bottom 96-well high binding plates (NUNC-MaxiSorp, Thermo Fisher Scientific) were coated with 1 μg/ml of histone H3 or H4 dissolved in 200 μl of carbonate coating buffer (50 mM, pH 9.6) per well at 4 °C overnight. The plates were washed three times with PBS containing 0.1% Tween-20 (PBST). The biotin-tagged spike (Sino Biological, 40589-V08B1-B), S1 subunit (Sino Biological, 40591-V08H-B), S2 subunit (Sino Biological, 40590-V08B-B), RBD (Sino Biological, 40592-V08B-B) or ACE2 proteins (Sino Biological, 10108-H27B-B) at concentrations of 0, 1, 5, 10, 25, 50, or 100 nM were added to each well and incubated at room temperature for 30 min. Each well was washed three times with PBST. Streptavidin-conjugated horseradish peroxidase (HRP) was added to each well and incubated for 30 min. Then, the wells were washed five times with 200 μl of PBST and developed with TMB substrate. The reaction was quenched by the addition of 50 μl of 1.0 M H2SO4 solution. The absorbance was measured at 450 nm. SPR-based measurements were performed by Biacore (8 K) as previously described [37]. Briefly, the histone H3 or H4 proteins were immobilized on a CM5 sensorchip (Cytiva) to a level of 25 response units (RUs) using Biacore (8 K). For affinity analysis, the spike protein (S1 + S2), S1 subunit or S2 subunit were dissolved in HBS-EP + running buffer at concentrations of 78.125, 156.25, 312.5, 625, and 1250 nM and were run across the chip. Each sample that was bound to the antigen surface was dissociated by HBS-EP+ buffer for 120 s at a flow rate of 30 μl/min. Regeneration of sensor chips was performed for 60 s using regeneration buffer (glycine pH 1.5). The dissociation constant (KD) was determined and recorded by Biacore (Cytiva). We used the rigid-body protein–protein docking algorithm ZDOCK [38] to systematically search the rotational and translational space of SARS-CoV-2 S protein and histone. The trimeric S protein of SARS-CoV-2 (PDB ID 6VSB) [39] was used as the receptor protein, and the histone (PDB ID 6HKT) [40] was used as the ligand protein. We then refined and evaluated the interaction energies of docked conformations using the ZRANK algorithm [41], which uses a CHARMm-based scoring function to calculate the energy of docked poses. The docked poses were filtered based on the ZRANK score. Two thousand structures were retained and clustered using the pairwise root mean square error (RMSD) as the distance measure. Finally, the docked poses were discarded if clashing between the histones and the S protein N-glycans was detected. An electrostatic potential map of the C-terminus of the SARS-CoV-2 S protein was generated from a crystal structure (PDB ID 6VSB) and visualized using Discovery Studio 3.1. For the calculation of the binding energy, molecular dynamics (MD) simulations were performed using NMAD [42]. The structures of proteins extracted from the thermodynamic equilibration trajectories were used to calculate the interaction binding energy and the energy contributions of the critical residues. The initial structures were prepared based on cryo-EM or X-ray crystal structures: SARS-CoV-2 S protein (PDB ID 6VSB), histone H3 and H4 (PDB ID 6HKT), and sialic acid (PDB ID 2CWG). The unresolved residues were constructed using Modeller 9.23 [43]. For the S protein mutant simulations, D1139P, E1144K, E1150S, and D1153S were constructed from cryo-EM structure 6VSB using Modeller. To allow sufficient optimization of the interaction models of histones and sialic acid, the sialic acid molecules were randomly placed inside the simulated systems as the starting models of MD simulations. All-atom MD simulations were performed in the NPT ensemble. All simulated models were immersed in a TIP3P water box with a 15 angstrom edge length. Constant pressure (P = 1 bar) and temperature (T = 300 K) were maintained using the Langevin piston coupling algorithm. The charmm 36 force field [44] was employed for the protein. The charmm CGenFF force field [45] was used for sialic acid. The charge states of protein ionizable groups were normalized to pH 7.0. Sodium (Na+) and chloride (Cl–) counterions were added to ensure global charge neutrality at a physiological concentration of 0.15 M using VMD. The SHAKE algorithm was used to constrain the lengths of all chemical bonds involving hydrogen atoms. The integration time step of the simulations was set to 2.0 fs. Nonbonded van der Waals interactions were treated by using a switching function at 10 Å. Long-range electrostatic forces were handled by using the particle mesh Ewald algorithm, which is an efficient method for periodic boundary conditions. The systems were minimized using the steepest-descents algorithm and heated from 50 K to 300 K with fixed protein backbone atoms. The systems were then submitted to NAMD for 10 ns NPT equilibrations before all-atom MD productions with the whole system relaxed. There are three acidic submotifs (1139–1153 aa, 1163–1168 aa, and 1184–1199 aa) in the acidic domain of S2. The potential histone-binding site calculated by the docking program was located in the first submotif. To verify whether this acidic submotif is the histone-binding site, we generated pseudoviruses carrying mutations (mut-1 and mut-2) in these submotifs by replacing the acidic amino acids with neutral or base residues. To prevent dramatic destruction of the native S protein structure, multiple sequence alignment of the coronavirus family members was performed using Clustal Omega to check the conservation level of mutation sites and to guide the amino acid substitution. Mut-1 covers the negatively charged amino acids and contains four point mutations (D1139P, E1144k, E1150S, and D1153S). Mut-2covers the other negatively charged amino acids, including seven point mutations (D1163A, D1165N, D1168S, D1184N, E1188Q, E1195N, and D1199N). Spike mutagenesis was performed with two-fragment PCR and Gibson-Assembly approach by using Gibson Assembly Master Mix (NEB, E2611) according to the instructions. Cell surface binding of H3 and H4 was detected by flow cytometry. Biotin-tagged histone H3 (10 ng/ml) or H4 (35 ng/ml) protein was added to Calu-3 cells in PBS buffer. After incubation at room temperature for 30 min, the cells were washed three times with PBS and then stained with a PE-conjugated antibody (Biolegend, MX2013931) (PE antibiotin) at 4 °C for 30 min. To block the binding of H3 or H4 to cells, H3 or H4 proteins were preincubated with Neu5Ac (10 μg/ml) or pretreated with NAs (0.1 U/ml) for 24 h. For the SARS-CoV-2 S2 subunit protein and cell surface binding assays, the biotin-tagged S2 subunit (5 nM) was preincubated with histone H3 or H4 at 37 °C for 20 min. The cells were then incubated with the mixture for 30 min. The cells were washed and stained with PE antibiotin antibody. Binding was detected by a flow cytometer (ACEA Biosciences), and the results were analyzed with FlowJo V10 software. In some experiments, a portion of the cells was pretreated with NAs (0.1 U/ml) in DMEM for 24 h at 37 °C. The establishment and detection of the SARS-CoV-2 S-mediated cell–cell fusion assays were described previously [3, 46]. Briefly, HEK-293T cells cotransfected with a plasmid encoding EGFP (pEGFP-C1) and a vector encoding the SARS-CoV-2 S glycoprotein with a C-terminal 18-aa truncation (293T/SARS-CoV-2 S/EGFP) were used as effector cells. 293T cells expressing human ACE2 receptors on the membrane surface (293T/ACE2) were utilized as target cells. Before the cell–cell fusion assays, the effector cells and target cells were treated with NA for 12 h. Effector cells and targeted cells were cocultured at a ratio of approximately one SARS-CoV-2 S protein-expressing cell to one ACE2 receptor-expressing cell in the absence or presence of histone H3 (1 μg/ml) or histone H4 (2 μg/ml) at the final concentration as indicated. After further coculture at 37 °C for 4 h, the cells were stained with Hoechst (Beyotime Biotechnology, Hoechst 33342), and syncytium formation between 293T/SARS-CoV-2/EGFP and 293T/ACE2 cells was observed under an inverted fluorescence microscope. 293T/EGFP cells were used as the negative control. Three fields were randomly selected in each well to count the number of fused and unfused cells. Transgenic hACE2 mice (8–10 weeks) on a C57BL/6 background were provided by the National Institutes for Food and Drug Control. The mouse model with the pathological changes of SARS-CoV-2-induced acute respiratory distress syndrome was established by intratracheal instillation, as previously described [33]. Briefly, the mice were anesthetized with 5% isoflurane, a small superficial incision was made in the midline of their necks to expose the trachea, and 4 × 105 PFU of SARS-CoV-2 in 60 μl of PBS was intratracheally instilled with a 29-gauge insulin syringe (Becton, Dickinson and Company, USA). In the total histone, histone H3 and histone H4 groups, 4 × 105 PFU of SARS-CoV-2 in 40 μl PBS was mixed with total histone, histone H3 or histone H4 at the indicated dose, and the final volume was kept at 60 μl. After incubation at 37 °C for 3 h, the mixtures were intratracheally instilled. After instillation, the overlying skin was closed with wound clips, and the animals were placed on a heating pad in their cage until they recovered from anesthesia. In the Cl-amidine- and Neu5Ac-treated groups, the mice were intraperitoneally (i.p.) injected with a total of 200 μl (50 mg/kg) of Cl-amidine or 200 μl (20 mg/kg) of Neu5Ac immediately after the SARS-CoV-2 challenge and were continuously injected once per day for five consecutive days. All mice were euthanized by isoflurane overdose 5 days post infection for serum collection and tissue processing. Lung tissues were collected for immunofluorescence (IF) staining and for RT-qPCR assays. All procedures associated with animal study were reviewed and approved by the Institutional Animal Care and Use Committee of Institute of Medical Biology, Chinese Academy of Medical Sciences, and were performed in the ABSL-4 facility of Kunming National High-level Biosafety Primate Research Center. SARS-CoV-2 E gene subgenomic mRNA, indicative of virus replication, was assessed by RT-qPCR as previously described [31, 47], using the following primer and probe sequences: forward, 5′-CGATCTCTTGTAGATCTGTTCTC-3′; reverse, 5′-ATATTGCAGCAGTACGCACACA-3′; probe, 5′-FAM-CGAAGCGCAGTAAGGATGGCTAGTGT-Quencher-3′. Lung tissues were harvested and used for IF staining [48]. Briefly, after incubation with blocking buffer (5% normal goat serum) for 10 min at room temperature, the slides were incubated with primary antibodies overnight at 4 °C. After five washes, the sections were incubated with secondary antibody at 37 °C for 1 h. In the in vitro experiment, neutrophils were allowed to attach to the coverslips and incubated for 4 h at 37 °C for immunostaining. The primary antibodies used for IF analysis included rabbit anti-MPO (Abcam, ab9535), rabbit anti-SARS-CoV-2 Spike (Sino Biological, 40589-T62), and recombinant anti-histone H3 (methyl K37) antibodies (Abcam, ab215728). Secondary antibodies included goat anti-rabbit IgG H&L (Alexa Fluor® 488) (Abcam) and goat anti-rat IgG H&L preadsorbed (Alexa Fluor® 647) (Abcam, ab150167). The TUNEL assay was performed according to the instructions of the DeadEndTM Fluorometric TUNEL System (Promega, USA). The results were observed by fluorescence microscopy (Leica, Germany). The number of positive cells was calculated from the observation of three random fields. The statistical analyses were carried out using Prism software (GraphPad Prism 8.0). Comparisons between two groups were performed using unpaired Student’s t tests. Comparisons among multiple groups were performed using one-way ANOVA followed by Tukey’s multiple comparison post hoc test. P < 0.05 was considered significant (significance is denoted as follows: ns no significance; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).Surface plasmon resonance analysis
Molecular modeling of SARS-CoV-2 S protein and histone
Spike mutagenesis
Binding of histone H3, H4, or S2 subunit proteins to the cell surface
Cell–cell fusion assay
Mice and experimental protocol
RT-qPCR assay
Immunofluorescence (IF) staining
TUNEL assay
Statistical analysis
Data availability
The study did not generate any unique datasets or codes.
References
Kim D, Lee JY, Yang JS, Kim JW, Kim VN, Chang H. The architecture of SARS-CoV-2 transcriptome. Cell. 2020;181:914–921. e910.
Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. e286.
**a S, Liu M, Wang C, Xu W, Lan Q, Feng S, et al. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30:343–55.
Chi X, Yan R, Zhang J, Zhang G, Zhang Y, Hao M, et al. A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2. Science. 2020;369:650–5.
Cantuti-Castelvetri L, Ojha R, Pedro LD, Djannatian M, Franz J, Kuivanen S, et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370:856–60.
Daly JL, Simonetti B, Klein K, Chen KE, Williamson MK, Antón-Plágaro C, et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020;370:861–5.
Clausen TM, Sandoval DR, Spliid CB, Pihl J, Perrett HR, Painter CD, et al. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell. 2020;183:1043–57.e1015.
Wei C, Wan L, Yan Q, Wang X, Zhang J, Yang X, et al. HDL-scavenger receptor B type 1 facilitates SARS-CoV-2 entry. Nat Metab. 2020;2:1391–400.
Brand SPC, Ojal J, Aziza R, Were V, Okiro EA, Kombe IK, et al. COVID-19 transmission dynamics underlying epidemic waves in Kenya. Science. 2021;374:989–94.
Wang K, Chen W, Zhang Z, Deng Y, Lian JQ, Du P, et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther. 2020;5:283.
Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew AA, et al. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol. 2011;179:199–210.
Saitoh T, Komano J, Saitoh Y, Misawa T, Takahama M, Kozaki T, et al. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe. 2012;12:109–16.
McNamara PS, Ritson P, Selby A, Hart CA, Smyth RL. Bronchoalveolar lavage cellularity in infants with severe respiratory syncytial virus bronchiolitis. Arch Dis Child. 2003;88:922–6.
Funchal GA, Jaeger N, Czepielewski RS, Machado MS, Muraro SP, Stein RT, et al. Respiratory syncytial virus fusion protein promotes TLR-4-dependent neutrophil extracellular trap formation by human neutrophils. PLoS ONE. 2015;10:e0124082.
Gwyer Findlay E, Currie SM, Davidson DJ. Cationic host defence peptides: potential as antiviral therapeutics. BioDrugs. 2013;27:479–93.
Hariton-Gazal E, Rosenbluh J, Graessmann A, Gilon C, Loyter A. Direct translocation of histone molecules across cell membranes. J Cell Sci. 2003;116:4577–86.
Kozlowski HN, Lai ETL, Havugimana PC, White C, Emili A, Sakac D, et al. Extracellular histones identified in crocodile blood inhibit in-vitro HIV-1 infection. AIDS. 2016;30:2043–52.
Rohrbach AS, Slade DJ, Thompson PR, Mowen KA. Activation of PAD4 in NET formation. Front Immunol. 2012;3:360.
Konig MF, Andrade F. A critical reappraisal of neutrophil extracellular traps and NETosis mimics based on differential requirements for protein citrullination. Front Immunol. 2016;7:461.
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5.
Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191:677–91.
Ermert D, Urban CF, Laube B, Goosmann C, Zychlinsky A, Brinkmann V. Mouse neutrophil extracellular traps in microbial infections. J Innate Immun. 2009;1:181–93.
Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19.
Tillack K, Breiden P, Martin R, Sospedra M. T lymphocyte priming by neutrophil extracellular traps links innate and adaptive immune responses. J Immunol. 2012;188:3150–9.
Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS ONE. 2012;7:e32366.
Middleton EA, He XY, Denorme F, Campbell RA, Ng D, Salvatore SP, et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136:1169–79.
Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, et al. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–21.
Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, et al. Neutrophil extracellular traps in COVID-19. JCI insight. 2020;5:e138999.
Radermecker C, Detrembleur N, Guiot J, Cavalier E, Henket M, d’Emal C, et al. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. J Exp Med. 2020;217:e20201012.
Veras FP, Pontelli MC, Silva CM, Toller-Kawahisa JE, de Lima M, Nascimento DC, et al. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J Exp Med. 2020;217:e20201129.
Yang J, Wang W, Chen Z, Lu S, Yang F, Bi Z, et al. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature. 2020;586:572–7.
Park YJ, Walls AC, Wang Z, Sauer MM, Li W, Tortorici MA, et al. Structures of MERS-CoV spike glycoprotein in complex with sialoside attachment receptors. Nat Struct Mol Biol. 2019;26:1151–7.
Hong W, Yang J, Bi Z, He C, Lei H, Yu W, et al. A mouse model for SARS-CoV-2-induced acute respiratory distress syndrome. Signal Transduct Target Ther. 2021;6:1.
Hoeksema M, Tripathi S, White M, Qi L, Taubenberger J, van Eijk M, et al. Arginine-rich histones have strong antiviral activity for influenza A viruses. Innate Immun. 2015;21:736–45.
Yuan M, Wu NC, Zhu X, Lee CD, So R, Lv H, et al. A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science. 2020;368:630–3.
Bestle D, Heindl MR, Limburg H, Van Lam van T, Pilgram O, Moulton H, et al. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci Alliance. 2020;3:e202000786.
Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–20.
Chen R, Li L, Weng Z. ZDOCK: an initial-stage protein-docking algorithm. Proteins. 2003;52:80–87.
Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–3.
Garcia-Saez I, Menoni H, Boopathi R, Shukla MS, Soueidan L, Noirclerc-Savoye M, et al. Structure of an H1-bound 6-nucleosome array reveals an untwisted two-start chromatin fiber conformation. Mol Cell. 2018;72:902–915. e907
Pierce B, Weng Z. ZRANK: reranking protein docking predictions with an optimized energy function. Proteins. 2007;67:1078–86.
Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, et al. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26:1781–802.
Martí-Renom MA, Stuart AC, Fiser A, Sánchez R, Melo F, Sali A. Comparative protein structure modeling of genes and genomes. Annu Rev Biophys Biomol Struct. 2000;29:291–325.
MacKerell AD, Bashford D, Bellott M, Dunbrack RL, Evanseck JD, Field MJ, et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J Phys Chem B. 1998;102:3586–616.
Vanommeslaeghe K, Hatcher E, Acharya C, Kundu S, Zhong S, Shim J, et al. CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J Comput Chem. 2010;31:671–90.
Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11:1620.
Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–9.
Sun SH, Chen Q, Gu HJ, Yang G, Wang YX, Huang XY, et al. A mouse model of SARS-CoV-2 infection and pathogenesis. Cell Host Microbe. 2020;28:124–33.e124.
Acknowledgements
We thank X. Wu, G. Wei, and H. Yan for pseudovirus generation and for providing cell culture, and thank Z. Li, X. Zhou, H. Yang, and Q. Chen for assisting with the maintenance of the mouse facility. This work was supported by the National Science Foundation for Excellent Young Scholars (32122052) and National Natural Science Foundation Regional Innovation and Development (No. U19A2003).
Author information
Authors and Affiliations
Contributions
XW, XP, SL, and AT conceived and supervised the research and designed the experiments. WH, CH, **gyun Yang, ZB, and HL performed the pseudovirus infection experiments and protein binding assays. JZ performed the molecular calculations. YY, WY, QH, MY, Y. Zhou, Y. Zhao, DK, JW, and HW performed the SARS-CoV-2 infection in vitro and mouse challenge experiments with live SARS-CoV-2. AT, KZ, and Zeng Wang constructed the plasmid and performed spike mutagenesis. AT, Zeng Wang, and WH performed the cell–cell fusion assays. XJ and **liang Yang performed SPR analysis. HL, XH, XL, AA, and WR performed immunofluorescence staining. XW, SC, ZH, ML, Z. Zhang, TL, Li Chen, HQ, WW, GS, GL, Z. Zhao, LY, XS, Zhenling Wang, JL, LD, CC, MG, Lu Chen, JG, WC, CH, YW, ZQ, and YP analyzed and interpreted the data and assisted with the adjustments of directions and interpretation of the mechanistic aspects of the results. XW wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hong, W., Yang, J., Zou, J. et al. Histones released by NETosis enhance the infectivity of SARS-CoV-2 by bridging the spike protein subunit 2 and sialic acid on host cells. Cell Mol Immunol 19, 577–587 (2022). https://doi.org/10.1038/s41423-022-00845-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-022-00845-6
- Springer Nature Limited
Keywords
This article is cited by
-
Cationic crosslinked carbon dots-adjuvanted intranasal vaccine induces protective immunity against Omicron-included SARS-CoV-2 variants
Nature Communications (2023)
-
The role of cell death in SARS-CoV-2 infection
Signal Transduction and Targeted Therapy (2023)
-
Circulating histones contribute to monocyte and MDW alterations as common mediators in classical and COVID-19 sepsis
Critical Care (2022)
-
Cell deaths: Involvement in the pathogenesis and intervention therapy of COVID-19
Signal Transduction and Targeted Therapy (2022)