Abstract
Tamoxifen-based endocrine therapy remains a major adjuvant therapy for estrogen receptor (ER)-positive breast cancer (BC). However, many patients develop tamoxifen resistance, which results in recurrence and poor prognosis. Herein, we show that fatty acid oxidation (FAO) was activated in tamoxifen-resistant (TamR) ER-positive BC cells by performing bioinformatic and functional studies. We also reveal that CPT1A, the rate-limiting enzyme of FAO, was significantly overexpressed and that its enzymatic activity was enhanced in TamR cells. Mechanistically, the transcription factor c-Jun was activated by JNK kinase-mediated phosphorylation. Activated c-Jun bound to the TRE motif in the CPT1A promoter to drive CPT1A transcription and recruited CBP/P300 to chromatin, catalysing histone H3K27 acetylation to increase chromatin accessibility, which ensured more effective transcription of CPT1A and an increase in the FAO rate, eliminating the cytotoxic effects of tamoxifen in ER-positive BC cells. Pharmacologically, inhibiting CPT1A enzymatic activity with the CPT1 inhibitor etomoxir or blocking c-Jun phosphorylation with a JNK inhibitor restored the tamoxifen sensitivity of TamR cells. Clinically, high levels of phosphorylated c-Jun and CPT1A were observed in ER-positive BC tissues in patients with recurrence after tamoxifen therapy and were associated with poor survival. These results indicate that the assessment and targeting of the JNK/c-Jun-CPT1A-FAO axis will provide promising insights for clinical management, increased tamoxifen responses and improved outcomes for ER-positive BC patients.
Similar content being viewed by others
Introduction
Breast cancer (BC) is the most frequently diagnosed cancer and ranks as the second most common cause of cancer-related death in females [1]. BCs are classified according to the expression status of hormone receptors and human epidermal growth factor receptor. Estrogen receptor (ER)-positive BC is the most common BC subtype, accounting for approximately 80% of all BC cases [2]. Endocrine therapy that suppresses estrogen production or targets ER is widely utilised as an adjuvant treatment for patients with ER-positive BC [3]. Tamoxifen is widely used as the standard first-line endocrine therapy, and tamoxifen therapy can reduce cancer recurrence and mortality. However, as many as 40% of patients ultimately develop tamoxifen resistance [4, 5]. Tamoxifen resistance has thus become a major challenge limiting therapy outcomes for ER-positive BC patients.
Both de novo and acquired resistance to tamoxifen occur in patients with BC, and the latter is more commonly used to explain recurrence after long-term tamoxifen therapy in the clinic. In most cases, tamoxifen resistance occurs as a result of genetic or epigenetic alterations in various components of signalling pathways, such as gain-of-function mutations in the ER, altered interactions of the ER with coactivators or corepressors, and compensatory crosstalk between ER and oncogenic signalling pathways [6]. Nonetheless, the molecular mechanism underlying tamoxifen resistance, especially acquired resistance, remains to be further clarified, and this clarification may improve patient responsiveness to clinical treatment.
Tumour cell reprogramming of cellular metabolism supports the molecular interpretation of the malignant biological behaviour of cancer [7]. In addition to glucose and glutamine metabolism, fatty acid metabolism provides large amounts of energy in tumour cells. De novo synthesis of fatty acids supports membrane synthesis for cell proliferation, and fatty acid catabolism mediated via fatty acid oxidation (FAO; also known as β-oxidation) provides more ATP and NADPH than are produced from carbohydrates to enable cell survival [8]. Aberrant activation of FAO is needed for tumour cells to maintain stemness, fuel tumour growth, initiate metastasis, develop drug resistance and evade the immune response [Full size image
FAO is a primary source of cell energy, as it yields ATP and cytosolic NADPH, but limited studies have focused on FAO rather than the well-recognised Warburg effect [20]. Recently, FAO has been shown to be a critical inducer of BC stem cell self-renewal and chemoresistance and has been shown to drive BC cell metastasis [10, 21, 22], but its role in tamoxifen resistance in the BC context remains unclear. To clarify the changes in the FAO process in tamoxifen-resistant ER-positive BC cells, we measured FAO activity and ATP production in TamR cells and parental cells. We found that the enzymatic activity of carnitine palmitoyl-transferase 1 (CPT1), the rate-limiting enzyme in FAO, was significantly enhanced in TamR cells (Fig. 1c), and the FAO rate and ATP production rates were accelerated (Fig. 1d, e). Since the expression of CPT1 isoforms exhibits tissue- and cell type-specific patterns [23], we sought to identify the isoform that was altered in TamR cells. The results showed that of the three isoforms of CPT1, only CPT1A was upregulated at both the mRNA and protein levels in TamR cells compared to parental cells (Fig. 1f, g). Immunofluorescence staining of CPT1A in the cytoplasm also indicated increased CPT1A expression in TamR cells (Fig. 1h). In addition, we found that the ectopic expression of CPT1A resulted in a reduction in tamoxifen sensitivity in wild-type ER-positive BC cells (Supplementary Fig. 1c, d). These results indicated that CPT1A-mediated FAO induces tamoxifen resistance.
Etomoxir (ETX), an irreversible CPT1 inhibitor for FAO inhibition, was utilised to investigate the contribution of FAO in inducing tamoxifen resistance in BC cells. ETX treatment decreased the enzymatic activity of CPT1 in an ETX dose-dependent manner and restored tamoxifen sensitivity in two TamR cell lines (Fig. 1i, j and Supplementary Fig. 1e, f), which confirmed that CPT1 activity is required for the development of tamoxifen resistance. Taken together, these data support the finding that CPT1A-mediated FAO activation is a key driver of tamoxifen resistance in ER-positive BC cells.
c-Jun deletion restores tamoxifen sensitivity
Since CPT1A was significantly upregulated at the transcriptional level in TamR cells, we next explained this finding in terms of transcriptional regulation. To identify the candidate transcription factor (TF) that induces FAO activation in endocrine-resistant BC cells, we performed a TF enrichment analysis for the 334 upregulated genes in TamR cells by using the ChEA3 web server [24]. The results showed that the majority of these upregulated genes was coregulated by the TF c-Jun (Fig. 2a). We confirmed that c-Jun expression was markedly increased in TamR cells compared to parental cells (Fig. 2b).
a Top five transcription factors that regulate the expression of upregulated genes in TamR cells, as analysed by the ChEA3 web server. b Western blot assessment of c-Jun protein levels in TamR and parental MCF7 and T47D cells. c Pearson correlations between c-Jun mRNA levels and tamoxifen sensitivities in pan-cancer and BC cell lines. Pearson R = 0.129 for pan-cancer cell lines, and Pearson R = 0.286 for BC cell lines. AUC area under curve; a higher AUC indicates stronger cell viability under tamoxifen treatment; TPM transcripts per million. d Western blot experiments to validate the c-Jun knockout efficiency in both TamR and parental MCF7 and T47D cells. e CCK-8 analysis of TamR and parental MCF7 and T47D cells with or without c-Jun knockout treated with a concentration gradient of tamoxifen for 72 h. f Representative images of colony formation assays in TamR and parental MCF7 cells with or without c-Jun knockout treated with tamoxifen (10 μM) or vehicle and stained with crystal violet. g Quantification results of the colony formation assays in (f). Unpaired Student’s t test in (g); *P < 0.05, **P < 0.01, ***P < 0.001.
c-Jun is a component of the AP-1 transcription factor family that regulates target gene transcription to deregulate cancer-relevant signalling pathways. The oncogenic role of c-Jun in the malignant phenotype of BC has been recognised [25]. Next, we analysed the biological connection between c-Jun and tamoxifen resistance in ER-positive BC cells. The corresponding association between gene expression and drug sensitivity obtained from the Cancer Dependency Map (https://depmap.org/portal/) showed that higher c-Jun expression was correlated with greater tamoxifen resistance in both pancancer and BC cell lines (Fig. 2c).
To directly determine the association between c-Jun expression and tamoxifen resistance in ER-positive BC cells, we deleted endogenous c-Jun expression in both TamR and parental cells via the CRISPR/Cas9 approach (Fig. 2d). The cell viability assay and colony formation assay confirmed that knockout of c-Jun enhanced the tamoxifen mediated cytotoxic effect in parental cells and also remarkably restored tamoxifen sensitivity in TamR cells, which reached the level similar to that in parental cells (Fig. 2e–g and Supplementary Fig. 2a, b). In addition, we found that c-Jun deletion slightly decreased the proliferative ability of ER-positive BC cells (Fig. 2f, g). Taken together, these results demonstrate that c-Jun is a key oncogene in the induction of tamoxifen resistance and tumour growth arrest in ER-positive BC.
c-Jun activates FAO to induce tamoxifen resistance
To confirm the link between c-Jun and FAO in the tamoxifen resistance of ER-positive BC cells, we overexpressed c-Jun in wild-type MCF7 and T47D cells (Fig. 3a). We found that ectopically expressed c-Jun enhanced CPT1 enzymatic activity, accelerated the FAO rate, and promoted ATP production (Fig. 3b–d). Moreover, c-Jun overexpression abrogated the tamoxifen-mediated growth-inhibitory effects in ER-positive BC cells (Fig. 3e), which suggested that c-Jun contributes to tamoxifen resistance by enhancing FAO activity.
a Western blot analysis to detect the c-Jun overexpression in both wild-type MCF7 and T47D cells. b–d Comparison of CPT1 enzymatic activities (b), FAO rates (c), and cellular ATP levels (d) in wild-type MCF7 and T47D cells transfected with c-Jun overexpressing construct or empty vector. e CCK-8 analysis of c-Jun or empty vector transfected wild-type MCF7 and T47D cells which were treated with a concentration gradient of tamoxifen for 72 h. f CPT1 enzymatic activities in c-Jun-overexpressing wild-type MCF7 cells with or without ETX treatment for 24 h. g CCK-8 analysis of c-Jun-overexpressing wild-type MCF7 cells treated with a concentration gradient of tamoxifen combined with or without ETX for 72 h. Unpaired Student’s t test in (b–d, f); **P < 0.01, ***P < 0.001.
Then, we treated c-Jun-overexpressing cells with the FAO inhibitor ETX to determine whether c-Jun-mediated activation of FAO was required for tamoxifen resistance. The results showed that ETX treatment blocked the c-Jun-mediated increase in CPT1 activity and reversed the enhancement of tamoxifen resistance mediated by c-Jun (Fig. 3f, g and Supplementary Fig. 3a, b). Together, these data provide the biological connection between c-Jun and FAO and confirm that FAO is critical for c-Jun-induced tamoxifen resistance in ER-positive BC cells.
c-Jun recruits CBP/P300 to activate CPT1A transcription
c-Jun can form homodimers or heterodimers with other AP-1 members to bind the cAMP-responsive element (CRE) or TPA-responsive element (TRE) and thus regulate the transcription of specific genes [26]. As predicted via use of the JASPAR database, a TRE motif (5′-TGACTCA-3′) at −815 to −809 bp upstream of the transcription start site (TSS) in the CPT1A promoter was identified (Supplementary Fig. 4a), which suggested that c-Jun might directly bind to the CPT1A promoter and activate CPT1A transcription to enhance FAO. As validated by protein and mRNA studies, c-Jun deletion inhibited CPT1A expression at the transcriptional level (Fig. 4a, b).
a, b Western blot and RT-qPCR assessment of the protein (a) and mRNA (b) levels of CPT1A in wild-type MCF7 and T47D cells with or without c-Jun knockout. c Schematic diagram of the TRE motif location on the wild-type (WT) or mutant (MUT) promoter of CPT1A for luciferase reporter assays. TRE, TPA-responsive element. d HEK293T cells were transfected with empty vector or c-Jun-overexpressing plasmid to analyse the luciferase reporter activity driven by the WT or MUT CPT1A promoter. e Chromatin immunoprecipitation quantitative PCR (ChIP-qPCR) analysis of c-Jun occupancy on the CPT1A promoter region around the TRE motif in TamR and parental MCF7 and T47D cells. f STRING analysis of proteins interact with c-Jun. g Immunoprecipitation assay to confirm the interaction between c-Jun and CBP/P300 in T47D cells. h ChIP-qPCR assessment for the comparison of CBP, P300, and H3K27ac occupancy on the CPT1A promoter in MCF7 cells with or without c-Jun knockout. i HEK293T cells were transfected with empty vector, c-Jun-, CBP-, or P300-overexpressing plasmid alone or together as indicated to analyse the luciferase reporter activity driven by the WT CPT1A promoter. Unpaired Student’s t test in (b, e, h), and paired Student’s t test in (d, i); **P < 0.01, ***P < 0.001, n.s., not significant.
To confirm the binding ability of c-Jun to the TRE motif in the CPT1A promoter, we generated luciferase reporter constructs containing the wild-type TRE motif or a mutant form to which c-Jun could not bind and then performed luciferase reporter assays (Fig. 4c). The results showed that the wild-type CPT1A promoter induced a substantial increase in luciferase activity with ectopic expression of c-Jun, while the mutant form completely inhibited the increase in activity induced by c-Jun overexpression (Fig. 4d). In addition, chromatin immunoprecipitation quantitative PCR (ChIP-qPCR) assays confirmed that the occupancy of c-Jun on the CPT1A promoter was significantly increased in TamR cells (Fig. 4e). These data suggest that the increase in CPT1A expression is mediated by the direct binding of c-Jun to the TRE motif within the promoter to drive CPT1A transcription.
TFs usually collaborate with epigenetic modifications to activate or repress target gene transcription. According to a STRING database analysis, c-Jun functionally interacted with P300 (Fig. 4f); an immunoprecipitation assay showed that c-Jun interacted with CBP/P300 to form a complex in ER-positive BC cells (Fig. 4g). CBP/P300 is a histone acetyltransferase that catalyses the acetylation of histone 3 lysine 27 (H3K27ac), which is a hallmark of active transcription [27]. Functionally, c-Jun deletion prevented CBP/P300 binding to the CPT1A promoter, which was followed by a reduction in the H3K27ac level around the TRE motif within the CPT1A promoter (Fig. 4h). Consistently, CBP/P300 synergised with c-Jun to enhance the transcriptional activity of the CPT1A promoter, but this effect was diminished when c-Jun was deleted (Fig. 4i and Supplementary Fig. 4b). These results confirmed that c-Jun is required for CBP/P300 binding to the CPT1A promoter and that c-Jun collaborates with H3K27ac mark-related signalling to prime CPT1A transcription.
JNK-dependent c-Jun phosphorylation activates FAO
Phosphorylation is required for the transactivation activity of c-Jun. In particular, the JNK family of MAP kinase phosphorylates c-Jun at Ser-63, resulting in translocation of c-Jun to the nucleus and the binding of c-Jun to chromatin for induction of target gene transcription [28]. Moreover, we found that activated JNK cascade signalling was significantly enriched in TamR cells (Supplementary Fig. 5a). Therefore, we next investigated the possible role of c-Jun phosphorylation in the tamoxifen resistance of ER-positive BC cells. Phosphorylated c-Jun at Ser-63 was significantly enriched in the nucleus of TamR cells (Fig. 5a, b and Supplementary Fig. 5b). Therefore, we generated a loss-of-function c-Jun S63A construct that cannot be phosphorylated and transfected it into MCF7 and T47D cells (Fig. 5c and Supplementary Fig. 5c). In contrast to the c-Jun wild-type construct, the c-Jun S63A mutant form failed to promote CPT1A expression at either the mRNA or protein level (Fig. 5c, d and Supplementary Fig. 5c, d); furthermore, the c-Jun S63A mutant failed to increase ATP production and the FAO rate in ER-positive BC cells (Fig. 5e, f and Supplementary Fig. 5e, f). In addition, the c-Jun S63A construct induced only minor effects on the promoter activity of CPT1A compared to profound effects mediated by the c-Jun wild-type construct (Fig. 5g). Moreover, wild-type c-Jun induced tamoxifen resistance in MCF7 cells, but the c-Jun S63A mutant exerted little effect on tamoxifen sensitivity in MCF7 cells (Fig. 5h).
a Representative images for immunofluorescence analysis of phosphorylated c-Jun at the Ser63 site (pS63-c-Jun, red) in TamR and parental T47D cells. DAPI (blue) served as a marker for nuclei. b Western blot analysis showing the different levels of pS63-c-Jun in the cytoplasm and nuclei between TamR and parental MCF7 cells. c–f Comparison of CPT1A protein levels (c), CPT1A mRNA levels (d), cellular ATP levels (e), and FAO rates (f) in MCF7 cells transfected with empty vector, wild-type c-Jun construct (WT), or Ser63 phosphorylation disabled mutant form of c-Jun construct (S63A). g HEK293T cells were transfected with CPT1A luciferase reporter plasmids together with empty vector, WT c-Jun, or S63A c-Jun construct to analyse the luciferase reporter activity of the CPT1A promoter. h Representative images (left) and quantification results (right) of the colony formation assays for MCF7 cells transfected with empty vector, WT c-Jun, or S63A construct and treated with tamoxifen (10 μM) or vehicle. i Western blot assessment of the protein levels of pS63-c-Jun, total c-Jun, and JNK in nuclei of MCF7 cells treated with a concentration gradient of the JNK inhibitor SP600125 for 24 h. Unpaired Student’s t test in (d–f, h), and paired Student’s t test in (g); **P < 0.01, ***P < 0.001.
In addition, with the application of SP600125, a pan JNK inhibitor (JNKi), the protein levels of c-Jun and Ser-63-phosphorylated c-Jun in the nucleus were markedly decreased in a dose-dependent manner in MCF7 cells, suggesting that JNK activity is needed for c-Jun activation in ER-positive BC cells (Fig. 5i). These results support the finding that JNK-dependent c-Jun phosphorylation activates FAO and tamoxifen resistance in ER-positive BC cells.
Blocking JNK/c-Jun increases tamoxifen sensitivity via FAO inhibition
Since JNKi inhibits the phosphorylation of c-Jun, we next evaluated whether JNKi attenuates FAO in ER-positive BC cells. Compared to vehicle treatment, treatment with SP600125 significantly reduced the CPT1 activity and FAO rate in MCF7 and T47D cells (Fig. 6a, b), which demonstrated that JNK-mediated c-Jun phosphorylation modulated the activity of FAO in ER-positive BC cells. Thus, we examined the potential synergistic effect of a JNKi and tamoxifen. As expected, combination treatment of SP600125 and tamoxifen sensitised MCF7 and T47D cells to the effects of tamoxifen (Fig. 6c). A Chou–Talalay assay confirmed the synergistic effects of tamoxifen and SP600125 in MCF7 and T47D cells (Fig. 6d). Moreover, SP600125 treatment decreased TamR cell viability in vitro (Fig. 6e).
a, b Comparison of CPT1 enzymatic activities (a) and FAO rates (b) in MCF7 and T47D cells treated with the JNK inhibitor SP600125 (10 μM) or vehicle for 24 h. c Cell viability analysis for MCF7 and T47D cells treated with a concentration gradient of tamoxifen combined with SP600125 (10 μM) or vehicle for 72 h. IC50, half maximal inhibitory concentration. d Combination index-fraction affected plots of combined treatment of tamoxifen and SP600125 in MCF7 and T47D cells. Plots were generated using CompuSyn software. Combination index (CI) < 1, CI = 1, and CI > 1 indicate synergism, additive effect, and antagonism, respectively. A smaller CI value indicates stronger synergism. e CCK-8 analysis for TamR and parental MCF7 and T47D cells treated with a concentration gradient of tamoxifen combined with SP600125 (10 μM) treatment or not for 72 h. f Representative data of tumours in nude mice bearing MCF7-TamR cells received different treatment, n = 6/group. g Statistical analysis of mouse tumour weight in different groups, n = 6/group. h Representative immunohistochemistry (IHC) staining for Ki-67 (upper) and pS63-c-Jun (lower) in formalin-fixed tumour sections from the indicated treatment groups. Scale bars = 50 μm. Unpaired Student’s t test in (a–c, g); ***P < 0.001.
To further determine the efficacy of SP600125 in the growth arrest in TamR cell tumours in vivo, we subcutaneously implanted MCF7 TamR cells into athymic nude mice. There was little significant reduction in tumour weight in the TamR tumour-bearing group that underwent tamoxifen treatment, but SP600125 treatment diminished tumour growth and inhibited the phosphorylation of c-Jun in vivo (Fig. 6f–h). The combination of tamoxifen and SP600125 significantly diminished tumour weight, tumour cell proliferation and c-Jun phosphorylation (Fig. 6f–h and Supplementary Fig. 6a) without inducing nephrotoxicity or liver toxicity (Supplementary Fig. 6b). Taken together, these data suggest that targeting JNK/c-Jun resensitises tamoxifen-resistant BC cells to tamoxifen therapy and abrogates tumour growth.
c-Jun predicts tamoxifen therapy outcomes in breast cancer patients
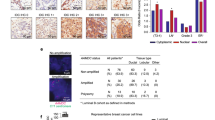
To further assess the relevance of c-Jun phosphorylation and CPT1A expression on the clinical outcomes of ER-positive BC patients, we assessed and quantified the expression of Ser-63-phosphorylated c-Jun and CPT1A by immunohistochemistry in ER-positive BC tissues. We found that high expression of pS63-c-Jun and CPT1A was more likely to be detected in patients with recurrence after tamoxifen therapy than in patients without recurrence (Fig. 7a, b). In addition, the expression level of pS63-c-Jun was positively correlated with CPT1A expression in ER-positive BC tissues (Fig. 7c and Supplementary Fig. 7a).
a Representative IHC staining of pS63-c-Jun (upper) and CPT1A (lower) proteins in tumour tissues from ER-positive BC patients received tamoxifen therapy with or without recurrence after. b Statistical analysis of the histoscore of pS63-c-Jun (upper) and CPT1A (lower) proteins in tumour tissues from ER-positive BC patients received tamoxifen therapy with or without recurrence. NR, non-recurrence; R, recurrence. Unpaired Student’s t test; **P < 0.01. c Correlations between pS63-c-Jun and CPT1A protein levels in ER-positive BC tissues. Pearson correlation coefficient for statistical analysis. d The heatmap illustrates the association of different clinical characteristics in ER-positive BC patients with high and low expression of pS63-c-Jun. e The heatmap illustrates the association of different clinical characteristics in ER-positive BC patients with high and low expression of CPT1A. Statistical significance was assessed by the Chi-square test in (d, e). f Recurrence-free survival (RFS) was compared between patients with high and low expression of pS63-c-Jun. g RFS was compared between patients with high and low expression of CPT1A. Log-rank test in (f, g); HR, Hazard ratio. h Model depicting the role of the JNK/c-Jun-CPT1A-FAO axis in driving tamoxifen resistance in ER-positive BC.
We next evaluated the relationship between the expression levels of pS63-c-Jun and CPT1A and the different clinicopathological features in patients with ER-positive BC. High pS63-c-Jun expression was correlated with advanced American Joint Committee on Cancer (AJCC) tumour node metastasis (TNM) stage and lymph node metastasis (Fig. 7d), and high CPT1A was also associated with lymph node metastasis (Fig. 7e). Finally, high levels of pS63-c-Jun and CPT1A were closely associated with shorter recurrence-free survival (RFS) and overall survival (OS) (Fig. 7f, g and Supplementary Fig. 7b, c). In addition, patients with high levels of both pS63-c-Jun and CPT1A showed a worsened prognosis after tamoxifen therapy than those with low expression of either one or both proteins (Supplementary Fig. 7d, e). These data indicate that phosphorylated c-Jun and CPT1A are pathologically and clinically associated with cancer recurrence and survival outcomes in ER-positive BC patients who received tamoxifen therapy.