Abstract
Human intestinal peptide transporter PEPT1 is commonly repressed in human colorectal cancer (CRC), yet its relationship with sensitivity to the common CRC treatment ubenimex has not previously been elucidated. In this study, we confirmed PEPT1 suppression in CRC using real-time quantitative polymerase chain reaction and western blotting and then investigated the underlying epigenetic pathways involved using bisulfite sequencing, chromatin immunoprecipitation, siRNA knockdown, and reporter gene assays. We found that PEPT1 transcriptional repression was due to both DNMT1-mediated DNA methylation of the proximal promoter region and HDAC1-mediated histone deacetylation, which blocked P300-mediated H3K18/27Ac at the PEPT1 distal promoter. Finally, the effects of the epigenetic activation of PEPT1 on the CRC response to ubenimex were evaluated using sequential combination therapy of decitabine and ubenimex both in vitro and in xenografts. In conclusion, epigenetic silencing of PEPT1 due to increased DNMT1 and HDAC1 expression plays a vital role in the poor response of CRC to ubenimex.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer and the second most deadly cancer worldwide, with an estimated over 1.9 million new cases and 935,000 deaths recorded in 20201. CRC incidence and mortality have declined with recent screening programs. However, the diagnosis rates of advanced CRC with low overall survival (OS) remain high1. Therefore, it is essential to identify novel biomarkers for the treatment, diagnosis, and prognosis of CRC. Chemotherapy is the treatment of choice to increase the OS of patients with advanced or metastatic CRC2. The resistance of malignant tumor cells to chemotherapy is a crucial reason for poor survival among CRC patients. One explanation for this multidrug resistance (MDR) to cancer chemotherapy is the increased efflux and reduced influx mediated by drug transporters3. For example, our laboratory’s previous studies have demonstrated that the repression of uptake transporters resulted in decreased drug concentrations in tumors4,5.
The peptide transporter PEPT1 (encoded by SLC15A1) is a prototypical member of the SLC15 family6. PEPT1 is predominantly responsible for the absorption of di/tripeptides and is mainly located on brush border membranes of small intestinal epithelia. In addition to that in the intestine, PEPT1 has been detected in tissues such as the nasal epithelium, kidney, biliary duct, and macrophages7,8. Besides, PEPT1 is overexpressed in the colon of inflammatory bowel disease patients and prostate cancer cells, which provides novel insight into the pathogenesis and tumor-specific drug delivery of this diseases9,10. However, there are inconsistent reports on the expression levels of PEPT1 mRNA and protein in the colon11. While one study reported low PEPT1 expression in the colon12, it could not be detected in other studies9,13. Another recent study showed that PEPT1 is highly expressed in the distal colon14. These inconsistent results may be explained at least partially by the different research groups measuring expression in different areas of the colon. PEPT1 has considerable substrate specificity for oligopeptides produced after the digestion of dietary or body proteins and structurally related drugs, such as the anticancer agent ubenimex (UBEN) and peptidomimetic prodrugs15. Ubenimex (UBEN), more commonly known as Bestatin, is a drug with immune-modulatory and anti-tumor activities16. At present, it has been widely used to treat acute myelocytic leukemia17 and it was once reportedly, preferably delivered into tumor cells overexpressing PEPT118. Therefore, PEPT1 is increasingly becoming a potential target for modulating the efficacy of various chemotherapeutic agents.
Until now, the transcriptional regulation of PEPT1 has not been widely investigated. Only a few transcription factors (sp1, cdx2, nrf2) have been shown to regulate the transcriptional activity of the PEPT1 gene promoter19,20,21. Epigenetic regulation of gene transcription, which involves dynamic modifications such as DNA methylation, histone acetylation, and noncoding RNAs, has been associated with several physiological and pathological processes. Although DNA methylation typically involved in gene repression is performed by DNA methyltransferase (DNMT1, DNMT3a, and DNMT3b), DNMT1 is primarily responsible for maintenance methylation by converting hemimethylated duplexes into symmetrically methylated CpG dinucleotides during DNA replication22,23. Alterations in DNA methylation caused by abnormalities in DNMT1 have been shown to drive tumorigenesis in several studies24,25. Histone acetylation primarily occurs at numerous lysine residues at the N terminus of histones, including H3K9Ac, H3K18Ac, and H3K27Ac, which are enriched around the transcription start site (TSS) and are generally associated with gene activation26,27. Acetyl groups are added at these specific histone sites by histone acetyltransferases (HATs) and removed by histone deacetylases (HDACs)28. The four main groups of HATs are the GCN5, MYST, CBP/P300, and SRC/p160 nuclear receptor coactivator families, and the HDAC family comprises eighteen different isoforms (HDAC1-11 and SIRT1-7)28. Similar to DNA methylation, histone acetylation at various gene transcriptional regulatory elements has been correlated with tumor development29. Epigenetic instability in CRC occurs early and manifests more frequently than genetic modifications30. For example, DNMT1-mediated methylation of Cdknla promoted cell proliferation in a carcinogen-administered CRC mouse model31. In addition, P300 was shown to promote PHF5A acetylation at K29 during cellular stress, which consequently contributed to colon carcinogenesis32 in the CRC xenograft model. Here, we determined the expression level of PEPT1 in CRC compared to normal tissues and analyzed the epigenetic mechanisms deregulating the expression of PEPT1 in CRC. We then designed a combination treatment of decitabine (DAC) and UBEN to sensitize CRC cells to UBEN. These findings highlight the potential clinical usefulness of various epigenetic modifications as biomarkers for the early diagnosis and pharmacological treatment of CRC patients.
Results
PEPT1 is repressed in CRC
Boxplot analysis on GEPIA revealed that PEPT1 transcription in CRC tumor tissues was markedly decreased compared to that in the normal colon (Fig. 1A). To confirm the mRNA expression levels of PEPT1, RT-qPCR was performed on 58 CRC tumor tissues and matched normal tissues (Fig. 1B, C). PEPT1 expression was dramatically reduced in most CRC tissue samples (43/58). Next, we evaluated the protein expression of PEPT1 via western blotting (Fig. 1D). Thirteen of the 14 CRC patient samples displayed low PEPT1 expression (Fig. 1B), whereas 1 sample (CRC47) displayed no significant change in protein expression. Moreover, no significant correlation was found between PEPT1 expression levels and gender, age, TNM stage, location (Fig. S1A–D, Table S1). These data together demonstrate that PEPT1 is downregulated at both the mRNA and protein levels in most CRC tumors, regardless of gender, age, and TNM stage. These results imply that the expression of PEPT1 is an important factor for the diagnosis of CRC.
A PEPT1 expression data were provided by TCGA in GEPIA. Box plots showed PEPT1 mRNA expression in CRC tumor tissues (T) and normal tissues (N). *P < 0.05, one-tailed unpaired t-test. B The mRNA expression of PEPT1 was detected in 58 pairs of CRC and corresponding adjacent non-tumorous tissues by RT-qPCR. Data are shown as means ± SD, n = 58. C The fold change log2(T/N) of PEPT1 expression between 58 pairs of CRC and adjacent normal tissues were summarized. <−1 is downregulation, −1 to 1 is unchanged and >1 is upregulation. D Representative images of western blotting analysis for the expression of PEPT1 in 14 paired samples of CRC. GAPDH was used as a loading control.
DNMT1 mediates the suppression of PEPT1
The silencing of gene expression by CpG methylation is one of the most frequent epigenetic inactivation events. Therefore, we next investigated whether DNA methylation at the PEPT1 promoter contributes to altered PEPT1 transcription in CRC. As shown in Fig. 2A, we found that treatment with DAC, a demethylation reagent that blocks cellular DNA methyltransferases (DNMTs), activated PEPT1 transcription in SW480 and SW620 cells in a dose-dependent manner. Western blot analysis confirmed that DAC induced PEPT1 expression at the protein level in both SW480 and SW620 cells (Fig. 2B). These data together imply that PEPT1 expression is controlled by DNA demethylation. As DNA methylation is catalyzed by DNMTs, including DNMT1, DNMT3a, and DNMT3b, we next investigated which DNMTs repressed PEPT1 transcription in CRC. Among the three DNMTs, only DNMT1 was upregulated in CRC tissues compared with normal tissues (Figs. 2C, S2), which indicated that DNMT1 might be a crucial factor responsible for PEPT1 repression. To investigate this further, we designed siRNAs against all 3 DNMTs to determine their role in regulating PEPT1. Interestingly, after transient transfection with siRNA, we found that PEPT1 mRNA and protein expression was upregulated by siDNMT1, but not siDNMT3a or siDNMT3b, in both SW480 and SW620 cells (Figs. 2D and E, S3). Furthermore, the ChIP results demonstrated that DNMT1 was absent at the PEPT1 promoter after DAC treatment (Fig. 2F). As a control, DAC treatment showed no effect on the DNMT1 signature at the GAPDH promoter (Fig. S4A). Overall, our results indicate that DNMT1 is associated with transcriptional repression of PEPT1 in CRC cells.
A The mRNA expression of PEPT1 in SW480 and SW620 cells. Cells were treated with DMSO, 2.5 μM DAC, or 5 μM DAC for 72 h. Data are shown as means ± SD, n = 3, two-tailed unpaired t-test, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, significantly different from DMSO. B Immunoblotting confirmed DAC treatment in SW480 and SW620 cells. C DNMT1 mRNA levels in CRC tumors (T) and normal tissues (N) from the TCGA in GEPIA. *P < 0.05, one-tailed unpaired t-test. D Knockdown of DNMT1 activated PEPT1 mRNA expression in SW480 and SW620 cells, respectively. SiNC, cells transfected with negative control siRNA, siDNMT1#1, siDNMT1#2, two siRNAs for DNMT1. Data are shown as means ± SD, n = 3, two-tailed unpaired t-test, **P < 0.01, ***P < 0.001. E PEPT1 and DNMT1 protein expression after DNMT1 knockdown in SW480 and SW620 cells. F ChIP-qPCR analyses of DNMT1 enrichment at the proximal promoter of PEPT1 in SW480 and SW620 cells after DAC (5 μM for 72 h) treatment. Data are shown as means ± SD, two-tailed unpaired t-test, *P < 0.05, ***P < 0.001.
DNA hypermethylation of PEPT1
As shown in Fig. 3A, the upstream region around the TSS of PEPT1 contains a putative CpG island (CGI, 900 bp). Using TCGA analysis, we found that promoter methylation of PEPT1 is significantly increased in CRC tissues compared to normal tissues (Fig. 3B). The upstream CGI at the PEPT1 proximal promoter region (−264 bp to +36 bp) contains 35 CpG sites. To directly determine whether the promoter region of PEPT1 is subject to DNA hypermethylation, we next examined methylation status at the PEPT1 promoter by BSP. To rule out unbiased amplification, the PCR reaction was optimized by combined bisulfite restriction analysis (Fig. S5). Eleven pairs of CRC and matched adjacent non-tumor tissues were used in the BSP. CRC tissues with the repression of PEPT1 had higher levels of DNA methylation in the sequenced region (−264 bp to +36 bp) compared with adjacent non-tumor colonic tissue samples, especially in the 25th to 30th CpG sites, suggesting that hypermethylation occurs at the PEPT1 promoter in CRC (Fig. 3C). Next, we examined whether exogenous DNA demethylation affects DNA methylation of the PEPT1 promoter in CRC cells. The PEPT1 promoter was hypermethylated in SW480 and SW620 cells (Fig. 3D). Upon treatment with DAC, globally inhibiting DNA methylation, CRC cells had decreased DNA methylation levels in the 25th to 30th CpG sites. In addition, a similar effect on the DNA methylation levels after DNMT1 siRNA transfection was also observed (Fig. 3E). As shown in Fig. 3F, knockdown of DNMT1 could also decrease the relative luciferase activity, indicating that DNMT1 binds directly to the promoter of PEPT1. Thus, we concluded that DNA hypermethylation mediated by DNMT1 represses PEPT1 in CRC.
A PEPT1 promoter region (±2.5 kb) around 5’ regions adjacent to the TSS. CGI, CpG islands. BSP, bisulfite-sequencing PCR. B DNA methylation data of PEPT1 promoter in cancerous and adjacent non-tumorous tissues from the TCGA database (***P < 0.001). C BSP analysis of PEPT1 CGI in cancerous and adjacent non-tumorous tissues (n = 11). Methylation percentages of the 35 CpG loci in the sequenced region were calculated. 11 pairs of CRC tissues belong to tissues in Fig. 1B (CRC17, 19, 22, 26, 41, 47, 49, 50, 51, 52, 54). The y axis indicates the average methylation percentage of each CpG site calculated from patient tissues with PEPT1 repression. D–E Calculation of methylation percentages of PEPT1 promoter after DAC and siDNMT1 treatment in CRC cells. F Luciferase assay in DNMT1 knockdown CRC cells. Mock, cells transfected with negative control siRNA and PEPT1 promoter constructs. Si-DNMT1, cells transfected with siDNMT1#2 and PEPT1 promoter constructs. Data are shown as means ± SD, two-tailed unpaired t-test, *P < 0.05, **P < 0.01.
Histone hypoacetylation represses PEPT1 in CRC
Histone acetylation is another important form of epigenetic regulation. We next attempted to decipher how histone acetylation contributes to PEPT1 repression in CRC. We found that treatment with the HDAC inhibitors TSA and SAHA also strongly increased the mRNA and protein expression of PEPT1 (Fig. 4A, B). Next, we treated cells with siRNAs targeting all HDAC family classes and observed significant upregulation of PEPT1 only after knockdown of HDAC1 (Figs. 4C and D, S3). These data suggest that hypoacetylation at the PEPT1 promoter region caused by HDAC1 subsequently leads to the repression of PEPT1 in CRC. In addition, ChIP-qPCR analyses demonstrated that the activating signals H3K18Ac and H3K27Ac increased after SAHA treatment (Figs. 4E, S4B). To confirm this result, we analyzed the occupancy of H3K18Ac and H3K27Ac to the promoter region of PEPT1 in CRC samples. In five CRC tissues with repressed PEPT1, both H3K18Ac and H3K27Ac were decreased at the promoter region (Figs. 4F and G, S4C). Collectively, these results suggest that the absence of H3K18Ac and H3K27Ac combined with HDAC1-mediated deacetylation results in histone hypoacetylation at the PEPT1 promoter region and transcriptional silencing of PEPT1 in CRC.
A The mRNA expression of PEPT1 in SW480 and SW620 cells after HDAC inhibitors treatment. Cells were treated with DMSO, 1 μM SAHA for 48 h or 0.5 μM TSA for 24 h. Data are shown as means ± SD, n = 3, two-tailed unpaired t-test, ns, no significance, **P < 0.01, ***P < 0.001, ****P < 0.0001. B Immunoblotting confirmed HDAC inhibitors treatment in SW480 and SW620 cells. C–D Knockdown of HDAC1 activated PEPT1 mRNA and protein expression in SW480 and SW620 cells, respectively. SiNC, cells transfected with negative control siRNA, siHDAC1#1, siHDAC1#2, two siRNAs for HDAC1. Data are shown as means ± SD, n = 3, two-tailed unpaired t-test, *P < 0.05, **P < 0.01, ****P < 0.0001. E ChIP-qPCR analyses of H3K18/H3K27Ac occupancy at PEPT1 promoter in SW480 and SW620 cells after SAHA (1 μM for 48 h) treatment. Data are shown as means ± SD, two-tailed unpaired t-test, **P < 0.01, ***P < 0.001, ****P < 0.0001. F PEPT1 mRNA expression in CRC47, 50, 51, 53, 55. Data are shown as means ± SD, two-tailed paired t-test, **P < 0.01, ***P < 0.001. G ChIP-qPCR analyses of H3K18/K27Ac occupancy at the PEPT1 promoter in five paired CRC tissues. Data are shown as means ± SD, two-tailed unpaired t-test, ***P < 0.001, ****P < 0.0001.
PEPT1 repression via the HDAC1-CBP/P300 axis
We next attempted to determine the role of positive and negative regulators of histone acetylation in regulating PEPT1. The acetyl group is usually added to lysine residues by histone acetyltransferases and removed by one of the HDAC enzymes. We performed dual-luciferase gene reporter assays to study whether histone acetyltransferases could bind to the UTR of PEPT1 and regulate its expression. We transiently co-transfected CRC cells with five PEPT1 promoter constructs based on the pGL3 luciferase reporter (Fig. 5A) and siRNAs targeting the histone acetyltransferases CBP/P300, which are specifically required for H3K18Ac and H3K27Ac33,34. We observed that inhibition of P300, but not CBP, reduced luciferase reporter activity by approximately 65% specifically at the region from −1750 to +129 bp in both SW480 and SW620 cells (Figs. 5B and S6). The luciferase assay results showed that P300 is a crucial factor for PEPT1 regulation and that P300 trans-activation of the PEPT1 promoter depends on the GGGAGTG sequence35, a consensus DNA-binding sequence for P300 (Fig. 5A, B). In addition, significantly lower expression levels of PEPT1 were found after P300 depletion in SW480 cells. (Fig. 5C, D). ChIP analysis also revealed that, after SAHA treatment, H3K18Ac and H3K27Ac were unoccupied in P300-silenced SW480 cells but highly enriched in non-targeting siRNA-expressing cells (Figs. 5E, S4D). In addition, H3K18Ac and H3K27Ac around the PEPT1 promoter were induced following knockdown of HDAC1 (Figs. 5F, S4E). These results together provide evidence that the HDAC1-CBP/P300 axis catalyzes H3K18Ac and H3K27Ac modification at the PEPT1 promoter.
A Promoter constructs of PEPT1 in the luciferase assay. GGGAGTG, a consensus DNA binding sequence for p300. B Luciferase assay in P300 knockdown CRC cells. Mock cells transfected with negative control siRNA and promoter constructs. Si-P300, cells transfected with siP300#2 and promoter constructs. Data are shown as means ± SD, n = 3, two-tailed unpaired t-test, ns, no significance, *P < 0.05, **P < 0.01. C–D Inhibition of P300 downregulated PEPT1. SiNC, cells transfected with negative control siRNA, siP300#1, siP300#2, two siRNAs for P300. Data are shown as means ± SD, n = 3, two-tailed unpaired t-test, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. E ChIP-qPCR analysis showed the effect of P300 expression on H3K18/K27Ac occupancy at the PEPT1 promoter. Cells were treated with DMSO or 1 μM SAHA for 48 h. SiNC, cells transfected with negative control siRNA, siP300, cells transfected with siP300#2. Data are shown as means ± SD, two-tailed unpaired t-test, ns, no significance, **P < 0.01, ***P < 0.001, ****P < 0.0001. F ChIP-qPCR analysis showed the effect of HDAC1 expression on H3K18/K27Ac occupancy at the PEPT1 promoter. SiNC, cells transfected with negative control siRNA, siHDAC1, cells transfected with siHDAC1#2. Data are shown as means±SD, two-tailed unpaired t-test, ***P < 0.001, ****P < 0.0001.
Sensitization of CRC cells to UBEN by PEPT1 activation
UBEN, a dipeptide analog, has been demonstrated to have diverse antitumor and immunomodulatory effects36,37. As UBEN is a typical PEPT1 substrate and appears to be transported into cells predominantly by PEPT138,39, the effects of UBEN are mediated by this oligopeptide transport activity18. Our earlier results showed that transcriptional repression of PEPT1 was associated with DNA methylation and that the demethylation reagent DAC induced PEPT1 expression. We, therefore, examined whether a combination treatment of DAC and UBEN enhanced cell death relative to either agent alone in vitro and in vivo. As shown in Fig. 6A, DAC greatly enhanced the cytotoxic effect of UBEN. Based on the Chou-Talalay method40, the sequential combination of DAC and UBEN resulted in a synergistic effect, with a combination index (CI) value less than 0.8 (Fig. 6B). DAC pretreatment lowered the IC50 values for UBEN from 106.82 to 2.09 μM (51-fold improvement) in SW480, and from an undetermined IC50 to 0.38 μM in SW620 (Fig. 6C). This result gives a new insight into how understanding PEPT1 epigenetic regulation allows a targeted approach to the synergistic drug combination of DAC and UBEN. Finally, the in vivo effects of combination therapy with DAC and UBEN were analyzed in SW480 and SW620 xenograft models. The timeline of the three cycles of combination chemotherapy is shown in Fig. 6D. PEPT1 expression was induced in xenograft tumors 7 days after the first DAC pretreatment (Fig. S7A and B). The administration of DAC resulted in weak tumor-suppressive effects. However, the sequential combination of DAC and UBEN led to strong tumor suppression effects, with a 50% reduction in tumor weight (Figs. 6E, F and S7D). In contrast, there were no significant changes in body weight between the treated and untreated groups (Fig. S7C). Collectively, these results demonstrate that DAC treatment, which induces epigenetic alteration of PEPT1, enhanced cellular accumulation and increased cytotoxicity of UBEN both in vitro and in vivo.
A Dose-effect curves of DAC, UBEN, and combination (DAC + UBEN) treatment in SW480 and SW620 cells. Cells were treated as indicated in Supplemental Table S2 and were subsequently analyzed using CCK8 assay. Data are shown as means ± SD, n = 6, nonlinear regression (curve fit) analysis. B Combination index (CI)–fraction affected (Fa) plots of DAC and UBEN combination were calculated by CompuSyn software in CRC cells. CI value is defined as follows: <0.8 is synergistic effect, from 0.8 to 1.2 is additive effect and >1.2 is antagonistic effect. C IC50 values of UBEN in CRC cells receiving combination treatment compared with UBEN alone. D Drug administration timeline and dosing schedule for xenograft models of CRC cells. E Relative tumor volume (RTV) curves in SW480 and SW620 xenograft models. Data represent the mean ± SD (n = 5). NC, UBEN, DAC, and DAC + UNEN indicate mice treated with sterile saline, ubenimex alone, decitabine alone, and decitabine-ubenimex combination, respectively. F Tumor weight of mice bearing SW480 and SW620 xenografts. Data represent means ± SD (n = 5), two-tailed unpaired t-test, ns, no significance, *P < 0.05, **P < 0.01.
Discussion
UBEN, a CD13/aminopeptidase N inhibitor, has been used in adjuvant chemotherapy as an excellent anticancer immunopotentiator and has been found to have cytotoxic effects in several cancer-cell lines41,42,43,44. Previous studies reported that combining UBEN with anticancer drugs, such as 5-FU, CDDP, and DXR, can reverse the resistance of various cancer cells to anticancer drugs by increasing intracellular ROS levels45. However, further mechanisms or signaling pathways underlying resistance to UBEN have not been clearly elucidated. Cancer cells have been shown to defend themselves against some chemotherapeutics, at least in part, by repression of the transporters responsible for their uptake4. In this study, we attempted to reverse drug resistance by targeting the epigenetic mechanisms that alter the expression of the transporter responsible for the uptake of UBEN into cancer cells.
PEPT1 is increasingly recognized as an important determinant of drug efficacy and a promising and attractive target in prodrug design15,46. In this study, our present data suggesting that the mRNA and protein levels of PEPT1 are decreased in CRC. Importantly, we found that DAC induced PEPT1 and enhanced the cytotoxicity of UBEN against human CRC cells, suggesting that PEPT1 plays a crucial role in chemotherapy resistance. It is also possible that other peptide transporters, such as PHT1, PHT2, and in particular PEPT2, which has a structural resemblance to PEPT1, may contribute to multidrug resistance in CRC47. However, PEPT2 is not found in the intestine and is instead highly expressed in renal proximal tubular cells47,48. In addition, DAC did not alter the expression level of PEPT2 in CRC cells (Fig. S8), indicating that UBEN is primarily transported by PEPT1, consequently resulting in high accumulation and increased cytotoxicity of UBEN after DAC treatment in CRC cells.
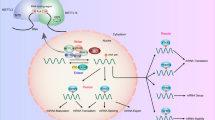
Our investigations into the epigenetic mechanisms underlying PEPT1 repression in CRC have indicated that both DNA methylation and histone acetylation regulate PEPT1 transcription (Fig. 7). We showed that a hypomethylated CpG island at the proximal promoter and occupancy of H3K18/27Ac at distal promoters leads to transcriptional activation of PEPT1 in the normal colorectum. CREB-binding protein (CBP) and its paralog P300 are histone acetyltransferases capable of acetylating H3K18, H3K27, H3K56, H3K14, and H3K2327,49. Previous studies have demonstrated that CBP and P300 act as histone acetyltransferase complexes due to their conserved sequence regions50,51. Some evidence also indicates that CBP and P300 perform unique functions52. For example, P300, but not CBP, independent of its histone acetyltransferase activity, is required to induce Tripartite motif 22 in IFNγ-mediated antiviral activity53. In mouse embryonic stem cells, only P300, and not CBP, is a critical factor for maintaining H3K27Ac at specific promoter regions of the genome54. In contrast, CBP (but not P300 or PCAF) is responsible for the hyperacetylation of DDX21, which impairs its helicase activity and leads to the accumulation of R loops and DNA damage55. Consistent with this, our results indicate that P300 is predominantly responsible for H3K18/27ac around the PEPT1 promoter. In addition, P300 contains an intrinsic and conserved DNA-binding domain, which shows a preferential affinity for the sequence GGGAGTG35. Our results confirmed that the element GGGAGTG (−1706 to 1701 bp) at the PEPT1 promoter contributed to the transcription of PEPT1. Future studies on P300 catalytic core domains such as PHD fingers may further decipher the details of histone acetylation at the PEPT1 promoter. Furthermore, we found that PEPT1 promoter repression in CRC is associated with a hypermethylated CpG island at the proximal promoter mediated by DNMT1 and the absence of H3K18/27Ac around the distal promoter due to HDAC1. Aberrant DNMT1 expression has been detected in CRC31, Chemical compounds studied in this article Decitabine (PubChem CID: 451668); Vorinostat (PubChem CID: 5311); Trichostatin A (PubChem CID: 444732); Ubenimex (PubChem CID: 72172). Key gene targets expressed in this article are hyperlinked to corresponding entries in the GEPIA2021 database (http://gepia2.cancerpku.cn/#analysis), which provides RNA sequencing (RNA-seq) data from 9736 tumors of The Cancer Genome Atlas (TCGA) database and 8587 normal samples of the Genotype-Tissue Expression (GTEx) database and offers tools for differential analysis, survival analysis, similar gene analysis, correlation analysis, and principal component analysis. Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. caac.21660 https://doi.org/10.3322/caac.21660 (2021). Dekker, E., Tanis, P. J., Vleugels, J. L. A., Kasi, P. M. & Wallace, M. B. Colorectal cancer. Lancet 394, 1467–1480 (2019). Li, W. et al. Overcoming ABC transporter-mediated multidrug resistance: molecular mechanisms and novel therapeutic drug strategies. Drug Resist. Updat. 27, 14–29 (2016). Liu, Y. et al. Epigenetic activation of the drug transporter OCT2 sensitizes renal cell carcinoma to oxaliplatin. Sci. Transl. Med. 8, 348ra97–348ra97 (2016). Ye, C. et al. Inhibition of histone deacetylase 7 reverses concentrative nucleoside transporter 2 repression in colorectal cancer by up-regulating histone acetylation state: CNT2 repression in colorectal cancer. Br. J. Pharmacol. 175, 4209–4217 (2018). Daniel, H. & Kottra, G. The proton oligopeptide cotransporter family SLC15 in physiology and pharmacology. Pflug. Arch. 447, 610–618 (2004). Agu, R. et al. Proton-coupled oligopeptide transporter (POT) family expression in human nasal epithelium and their drug transport potential. Mol. Pharm. 8, 664–672 (2011). Smith, D. E. et al. Tubular localization and tissue distribution of peptide transporters in rat kidney. Pharm. Res. 15, 1244–1249 (1998). Merlin, D. et al. Colonic epithelial hPepT1 expression occurs in inflammatory bowel disease: transport of bacterial peptides influences expression of MHC class 1 molecules. Gastroenterology 120, 1666–1679 (2001). Sun, D. et al. Expression of proton-coupled oligopeptide transporter (POTs) in prostate of mice and patients with benign prostatic hyperplasia (BPH) and prostate cancer (PCa). Prostate 73, 287–295 (2013). Wang, C.-Y., Liu, S., **e, X.-N. & Tan, Z.-R. Regulation profile of the intestinal peptide transporter 1 (PepT1). Drug Des. Dev. Ther. 11, 3511–3517 (2017). Ziegler, T. R. et al. Distribution of the H+/peptide transporter PepT1 in human intestine: up-regulated expression in the colonic mucosa of patients with short-bowel syndrome. Am. J. Clin. Nutr. 75, 922–930 (2002). Sai, Y. et al. Immunolocalization and pharmacological relevance of oligopeptide transporter PepT1 in intestinal absorption of beta-lactam antibiotics. FEBS Lett. 392, 25–29 (1996). Wuensch, T. et al. The peptide transporter PEPT1 is expressed in distal colon in rodents and humans and contributes to water absorption. Am. J. Physiol. Gastrointest. Liver Physiol. 305, G66–G73 (2013). Minhas, G. S. & Newstead, S. Structural basis for prodrug recognition by the SLC15 family of proton-coupled peptide transporters. Proc. Natl Acad. Sci. USA 116, 804–809 (2019). Ota, K. Review of ubenimex (Bestatin): clinical research. Biomed. Pharmacother. 45, 55–60 (1991). Sekine, K., Fujii, H. & Abe, F. Induction of apoptosis by bestatin (ubenimex) in human leukemic cell lines. Leukemia 13, 729–734 (1999). Nakanishi, T., Tamai, I., Takaki, A. & Tsuji, A. Cancer cell-targeted drug delivery utilizing oligopeptide transport activity. Int. J. Cancer 88, 274–280 (2000). Shimakura, J., Terada, T., Katsura, T. & Inui, K.-I. Characterization of the human peptide transporter PEPT1 promoter: Sp1 functions as a basal transcriptional regulator of human PEPT1. Am. J. Physiol. Gastrointest. Liver Physiol. 289, G471–G477 (2005). Shimakura, J., Terada, T., Shimada, Y., Katsura, T. & Inui, K. The transcription factor Cdx2 regulates the intestine-specific expression of human peptide transporter 1 through functional interaction with Sp1. Biochem. Pharmacol. 71, 1581–1588 (2006). Geillinger, K. E. et al. Nrf2 regulates the expression of the peptide transporter PEPT1 in the human colon carcinoma cell line Caco-2. Biochim. Biophys. Acta 1840, 1747–1754 (2014). Zhang, Z.-M. et al. Structural basis for DNMT3A-mediated de novo DNA methylation. Nature 554, 387–391 (2018). Jones, P. A. & Liang, G. Rethinking how DNA methylation patterns are maintained. Nat. Rev. Genet. 10, 805–811 (2009). Sen, G. L., Reuter, J. A., Webster, D. E., Zhu, L. & Khavari, P. A. DNMT1 maintains progenitor function in self-renewing somatic tissue. Nature 463, 563–567 (2010). Robert, M.-F. et al. DNMT1 is required to maintain CpG methylation and aberrant gene silencing in human cancer cells. Nat. Genet. 33, 61–65 (2003). Creyghton, M. P. et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl Acad. Sci. USA 107, 21931–21936 (2010). **, Q. et al. Distinct roles of GCN5/PCAF-mediated H3K9ac and CBP/p300-mediated H3K18/27ac in nuclear receptor transactivation. EMBO J. 30, 249–262 (2011). Verdin, E. & Ott, M. 50 years of protein acetylation: from gene regulation to epigenetics, metabolism and beyond. Nat. Rev. Mol. Cell Biol. 16, 258–264 (2015). Zhao, Z. & Shilatifard, A. Epigenetic modifications of histones in cancer. Genome Biol. 20, 245 (2019). Okugawa, Y., Grady, W. M. & Goel, A. Epigenetic alterations in colorectal cancer: emerging biomarkers. Gastroenterology 149, 1204–1225 (2015). e12. Luo, Y. et al. Intestinal PPARα protects against colon carcinogenesis via regulation of methyltransferases DNMT1 and PRMT6. Gastroenterology 157, 744–759 (2019). e4. Wang, Z. et al. Acetylation of PHF5A modulates stress responses and colorectal carcinogenesis through alternative splicing-mediated upregulation of KDM3A. Mol. Cell 74, 1250–1263 (2019). Raisner, R. et al. Enhancer activity requires CBP/P300 bromodomain-dependent histone H3K27 acetylation. Cell Rep. 24, 1722–1729 (2018). Peng, J. et al. p300/CBP inhibitor A-485 alleviates acute liver injury by regulating macrophage activation and polarization. Theranostics 9, 8344–8361 (2019). Rikitake, Y. & Moran, E. DNA-binding properties of the E1A-associated 300-kilodalton protein. Mol. Cell. Biol. 12, 2826–2836 (1992). Wang, X. et al. Role of ubenimex as an anticancer drug and its synergistic effect with Akt inhibitor in human A375 and A2058 cells. OncoTargets Ther. 11, 943–953 (2018). Jiang, Y. et al. Discovery of a novel chimeric ubenimex-gemcitabine with potent oral antitumor activity. Bioorg. Med. Chem. 24, 5787–5795 (2016). Wang, L. et al. PEPT1- and OAT1/3-mediated drug-drug interactions between bestatin and cefixime in vivo and in vitro in rats, and in vitro in human. Eur. J. Pharm. Sci. 63, 77–86 (2014). Jia, Y. et al. Enhancement effect of resveratrol on the intestinal absorption of bestatin by regulating PEPT1, MDR1 and MRP2 in vivo and in vitro. Int. J. Pharm. 495, 588–598 (2015). Chou, T.-C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 70, 440–446 (2010). Otsuki, T. et al. Aminopeptidase N/CD13 as a potential therapeutic target in malignant pleural mesothelioma. Eur. Respir. J. 51, 1701610 (2018). Terauchi, M. et al. Inhibition of APN/CD13 leads to suppressed progressive potential in ovarian carcinoma cells. BMC Cancer 7, 140 (2007). Umezawa, H., Aoyagi, T., Suda, H., Hamada, M. & Takeuchi, T. Bestatin, an inhibitor of aminopeptidase B, produced by actinomycetes. J. Antibiot. 29, 97–99 (1976). Muskardin, D. T., Voelkel, N. F. & Fitzpatrick, F. A. Modulation of pulmonary leukotriene formation and perfusion pressure by bestatin, an inhibitor of leukotriene A4 hydrolase. Biochem. Pharm. 48, 131–137 (1994). Yamashita, M. et al. A CD13 inhibitor, ubenimex, synergistically enhances the effects of anticancer drugs in hepatocellular carcinoma. Int. J. Oncol. 49, 89–98 (2016). Thompson, B. R., Shi, J., Zhu, H.-J. & Smith, D. E. Pharmacokinetics of gemcitabine and its amino acid ester prodrug following intravenous and oral administrations in mice. Biochem. Pharmacol. 180, 114127 (2020). Newstead, S. Recent advances in understanding proton coupled peptide transport via the POT family. Curr. Opin. Struct. Biol. 45, 17–24 (2017). Lu, X. et al. Human oligopeptide transporter 2 (PEPT2) mediates cellular uptake of polymyxins. J. Antimicrob. Chemother. 71, 403–412 (2016). Das, C., Lucia, M. S., Hansen, K. C. & Tyler, J. K. CBP/p300-mediated acetylation of histone H3 on lysine 56. Nature 459, 113–117 (2009). Manickavinayaham, S. et al. E2F1 acetylation directs p300/CBP-mediated histone acetylation at DNA double-strand breaks to facilitate repair. Nat. Commun. 10, 4951 (2019). Li, H.-M. et al. A potent CBP/p300-Snail interaction inhibitor suppresses tumor growth and metastasis in wild-type p53-expressing cancer. Sci. Adv. 6, eaaw8500 (2020). Henry, R. A., Kuo, Y.-M. & Andrews, A. J. Differences in specificity and selectivity between CBP and p300 acetylation of histone H3 and H3/H4. Biochemistry 52, 5746–5759 (2013). Gao, B. et al. p300, but not PCAF, collaborates with IRF-1 in stimulating TRIM22 expression independently of its histone acetyltransferase activity. Eur. J. Immunol. 43, 2174–2184 (2013). Martire, S., Nguyen, J., Sundaresan, A. & Banaszynski, L. A. Differential contribution of p300 and CBP to regulatory element acetylation in mESCs. BMC Mol. Cell Biol. 21, 55 (2020). Song, C., Hotz-Wagenblatt, A., Voit, R. & Grummt, I. SIRT7 and the DEAD-box helicase DDX21 cooperate to resolve genomic R loops and safeguard genome stability. Genes Dev. 31, 1370–1381 (2017). **e, J. et al. Metformin selectively inhibits metastatic colorectal cancer with the KRAS mutation by intracellular accumulation through silencing MATE1. Proc. Natl Acad. Sci. USA 117, 13012–13022 (2020). Imperiale, T. F. et al. Multitarget stool DNA testing for colorectal-cancer screening. N. Engl. J. Med. 370, 1287–1297 (2014). Lutz, L. et al. Histone modifiers and marks define heterogeneous groups of colorectal carcinomas and affect responses to HDAC inhibitors in vitro. Am. J. Cancer Res. 6, 664–676 (2016). Chen, C. et al. The histone deacetylase HDAC1 activates HIF1α/VEGFA signal pathway in colorectal cancer. Gene 754, 144851 (2020). Kim, S. L., La, M. T., Shin, M. W., Kim, S.-W. & Kim, H.-K. A novel HDAC1 inhibitor, CBUD-1001, exerts anticancer effects by modulating the apoptosis and EMT of colorectal cancer cells. Int. J. Oncol. https://doi.org/10.3892/ijo.2020.5109 (2020). Fuks, F., Burgers, W. A., Brehm, A., Hughes-Davies, L. & Kouzarides, T. DNA methyltransferase Dnmt1 associates with histone deacetylase activity. Nat. Genet. 24, 88–91 (2000). Li, L.-C. & Dahiya, R. MethPrimer: designing primers for methylation PCRs. Bioinformatics 18, 1427–1431 (2002). Eads, C. A. & Laird, P. W. Combined Bisulfite Restriction Analysis (COBRA). In DNA Methylation Protocols (eds. Mills, K. I. & Ramsahoye, B. H.) 71–86 (Springer, New York, 2002). Lee, T. I., Johnstone, S. E. & Young, R. A. Chromatin immunoprecipitation and microarray-based analysis of protein location. Nat. Protoc. 1, 729–748 (2006). Haring, M. et al. Chromatin immunoprecipitation: optimization, quantitative analysis and data normalization. Plant Methods 3, 11 (2007). Zhao, S. et al. Inhibition of LTA4H by bestatin in human and mouse colorectal cancer. EBioMedicine 44, 361–374 (2019). We would like to thank Editage (www.editage.cn) for English language editing. This work was financially supported by the National Natural Science Foundation of China (No. 81702801), China Postdoctoral Science Foundation (2020M680130), Zhejiang Provincial Natural Sciences Foundation of China (No. LY18H310012), Hangzhou City Scientific Technology Research Foundation of Zhejiang Province, China (No. 20180533B67), National Natural Science Foundation of China (No. 81872935). X.Z. and L.Y. conceived and designed the experiments. Y.W. and X.Z. wrote the manuscript. Y.W. and L.Q. performed the majority of the experiments and data analysis. J.W. and L.Y. conducted some experiments about Bisulfite sequencing analysis. Y.H., S.W. and S.Z. supervised and validated the paper. All authors have read and approved the final manuscript. This project was approved by the Institutional Review Board of Hangzhou Cancer Hospital (Permit Number: HZCH-2017-09). All animal experiments were approved by the Zhejiang University Animal Care and Use Committee (Ethics Code: ZJU20200062). The authors declare no competing interests. Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Edited by A. Woloszynska Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Wang, Y., Wang, J., Yang, L. et al. Epigenetic regulation of intestinal peptide transporter PEPT1 as a potential strategy for colorectal cancer sensitization.
Cell Death Dis 12, 532 (2021). https://doi.org/10.1038/s41419-021-03814-5 Received: Revised: Accepted: Published: DOI: https://doi.org/10.1038/s41419-021-03814-5Data availability
References
Acknowledgements
Funding
Author information
Authors and Affiliations
Contributions
Corresponding authors
Ethics declarations
Ethics statement
Conflict of interest
Additional information
Rights and permissions
About this article
Cite this article