Abstract
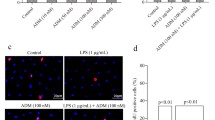
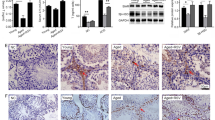
Adrenomedullin (ADM) exerts anti-oxidant, anti-inflammatory and anti-apoptotic effects in Leydig cells. However, the role and mechanism of ADM in the pyroptosis of Leydig cells are poorly understood. This study first showed the protective effects of ADM on the pyroptosis and biological functions of Leydig cells exposed to lipopolysaccharide (LPS) by promoting autophagy. Primary rat Leydig cells were treated with various concentrations of LPS and ADM, together with or without N-acetyl-L-cysteine (NAC) or 3-methyladenine (3-MA). Cell proliferation was detected through CCK-8 and BrdU incorporation assays, and ROS level was measured with the DCFDA assay. Real-time PCR, western blot, immunofluorescence, transmission electron microscopy, TUNEL and flow cytometry were performed to examine ADM’s effect on the pyroptosis, autophagy and steroidogenic enzymes of Leydig cells and AMPK/mTOR signalling. Like NAC, ADM dose-dependently reduced LPS-induced cytotoxicity and ROS overproduction. ADM also dose-dependently ameliorated LPS-induced pyroptosis by reversing the increased expression of NLRP3, ASC, caspase-1, IL-1β, IL-18, GSDMD, caspase-3, caspase-7, TUNEL-positive and PI and active caspase-1 double-stained positive rate, DNA fragmentation and LDH concentration, which could be rescued via co-incubation with 3-MA. ADM dose-dependently increased autophagy in LPS-induced Leydig cells, as confirmed by the increased expression of LC3-I/II, Beclin-1 and ATG-5; decreased expression of p62 and autophagosomes formation; and increased LC3-II/LC3-I ratio. However, co-treatment with 3-MA evidently decreased autophagy. Furthermore, ADM dose-dependently rescued the expression of steroidogenic enzymes, including StAR, P450scc, 3β-HSD and CYP17, and testosterone production in LPS-induced Leydig cells. Like rapamycin, ADM dose-dependently enhanced AMPK phosphorylation but reduced mTOR phosphorylation in LPS-induced Leydig cells, which could be rescued via co-incubation with 3-MA. In addition, pyroptosis was further decreased, and autophagy was further promoted in LPS-induced Leydig cells upon co-treatment with ADM and rapamycin. ADM may protect the steroidogenic functions of Leydig cells against pyroptosis by activating autophagy via the ROS–AMPK–mTOR axis.
Similar content being viewed by others
Introduction
Leydig cells, located within the interstitial compartment of the testes, mainly contribute to testosterone synthesis and secretion and play a principal role in the development of male traits, reproductive activity and male factor fertility1. Bacterial lipopolysaccharide (LPS) can induce oxidative stress that leads to the perturbation of Leydig cell mitochondria, which may be the major influential factor involved in the steroidogenic impairment of Leydig cells2,3,4. Therefore, understanding the cellular and molecular mechanisms underlying the recovery of steroidogenic impairment of Leydig cells has important implications.
The identification of key molecules in the recovery of impaired steroidogenic property of Leydig cells that can be targeted for therapy may help improve outcomes for patients with acute bacterial orchitis. One such potential molecule is adrenomedullin (ADM), which is a possible target for novel therapeutic intervention5. ADM is a 52-amino acid peptide originally discovered in human pheochromocytoma tissue and characterised by vasodilation and blood-pressure-lowering effects6. Given its anti-oxidant, anti-inflammatory, anti-apoptotic and proliferative properties, ADM exhibits potent protective functions under diverse pathological conditions as an endogenous peptideWestern blot After Leydig cells were treated in accordance with the above-described experimental design and reached confluence, the entire cell lysates were harvested from Leydig cell monolayers. Total protein was adjusted to equal amounts, and protein mixtures were separated via SDS-PAGE and transferred to polyvinylidene difluoride membranes. After the transfer, non-specific binding sites of the membranes were blocked for 1 h at room temperature in PBS (pH 7.4) containing 5% (wt/vol) non-fat dry milk and then incubated with primary antibodies against NLRP3 (1:1000), ASC (1:1000), caspase-1 (1:200), caspase-3 (1:200), caspase-7 (1:200), IL-1β (1:400), IL-18 (1:400), GSDMD (1:1000), LC3-I/II (1:1000), Beclin-1 (1:1000), ATG5 (1:500), p62 (1:500), StAR (1:1000), P450scc (1:1000), 3β-HSD (1:500), CYP17 (1:500), p-AMPK (1:1000), AMPK (1:1000), p-mTOR (1:1000) and mTOR (1:1000) at 4 °C overnight. The membranes were probed with an anti-β-actin antibody (1:1000) to control protein loading, and then incubated for 2 h at room temperature with HRP-conjugated secondary antibodies (1:1000). The results were scanned using a gel imaging system (UVP Company, Upland, CA, USA). Densitometry measurements were performed with Image Lab software (Bio-Rad Laboratories, Hercules, CA, USA). The band intensities were semi-quantified via densitometry analysis using Quantity-One software (Bio-Rad Laboratories, Hercules, CA, USA). Relative protein expression was normalised to β-actin and compared with the control group. Data were expressed as mean ± standard deviation on the basis of at least five separate experiments. Data were analysed using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). Significant differences amongst the mean values of multiple groups were evaluated with one-way ANOVA followed by Student–Newman–Keuls’ method. A two-sided P value < 0.05 was considered statistically significant. This study followed the national guidelines and protocols of the National Institutes of Health and was approved by the Local Ethics Committee for the Care and Use of Laboratory Animals of the University of South China.Statistical analysis
Ethical approval
References
Fijak, M. et al. Influence of testosterone on inflammatory response in testicular cells and expression of transcription factor Foxp3 in T cells. Am. J. Reprod. Immunol. 74, 12–25 (2015).
Allen, J. A., Diemer, T., Janus, P., Hales, K. H. & Hales, D. B. Bacterial endotoxin lipopolysaccharide and reactive oxygen species inhibit Leydig cell steroidogenesis via perturbation of mitochondria. Endocrine 25, 265–275 (2004).
Reddy, M. M. et al. Bacterial lipopolysaccharide-induced oxidative stress in the impairment of steroidogenesis and spermatogenesis in rats. Reprod. Toxicol. 22, 493–500 (2006).
Metukuri, M. R., Reddy, C. M., Reddy, P. R. & Reddanna, P. Bacterial LPS-mediated acute inflammation-induced spermatogenic failure in rats: role of stress response proteins and mitochondrial dysfunction. Inflammation 33, 235–243 (2010).
Marinoni, E. et al. Adrenomedullin in human male reproductive system. Eur. J. Obstet. Gynecol. Reprod. Biol. 122, 195–198 (2005).
Geven, C., Kox, M. & Pickkers, P. Adrenomedullin and adrenomedullin-targeted therapy as treatment strategies relevant for sepsis. Front. Immunol. 9, 292 (2018).
**an, X. et al. Vasoprotective activities of the adrenomedullin-RAMP2 system in endothelial cells. Endocrinology 158, 1359–1372 (2017).
Hu, W. et al. Adrenomedullin attenuates interleukin-1beta-induced inflammation and apoptosis in rat Leydig cells via inhibition of NF-kappaB signaling pathway. Exp. Cell Res. 339, 220–230 (2015).
Li, Y. Y., Hwang, I. S., O, W. S. & Tang, F. Adrenomedullin peptide: gene expression of adrenomedullin, its receptors and receptor activity modifying proteins, and receptor binding in rat testis–actions on testosterone secretion. Biol. Reprod. 75, 183–188 (2006).
Chan, Y. F., O, W. S. & Tang, F. Adrenomedullin in the rat testis. I: its production, actions on testosterone secretion, regulation by human chorionic gonadotropin, and its interaction with endothelin 1 in the Leydig cell. Biol. Reprod. 78, 773–779 (2008).
Zhou, P. H., Hu, W., Zhang, X. B., Wang, W. & Zhang, L. J. Protective effect of adrenomedullin on rat Leydig cells from lipopolysaccharide-induced inflammation and apoptosis via the PI3K/Akt signaling pathway ADM on rat Leydig cells from inflammation and apoptosis. Mediat. Inflamm. 2016, 7201549 (2016).
Hu, W. et al. Adrenomedullin protects Leydig cells against lipopolysaccharide-induced oxidative stress and inflammatory reaction via MAPK/NF-κB signalling pathways. Sci. Rep. 7, 16479 (2017).
Wallach, D., Kang, T. B., Dillon, C. P. & Green, D. R. Programmed necrosis in inflammation: toward identification of the effector molecules. Science 352, aaf2154 (2016).
Man, S. M., Karki, R. & Kanneganti, T. D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 277, 61–75 (2017).
Gonzalez, C. R., Muscarsel, Isla., M. L. & Vitullo, A. D. The balance between apoptosis and autophagy regulates testis regression and recrudescence in the seasonal-breeding South American plains vizcacha, Lagostomus maximus. PloS ONE 13, e0191126 (2018).
Bialik, S., Dasari, S. K. & Kimchi, A. Autophagy-dependent cell death—where, how and why a cell eats itself to death. J. Cell Sci. 131, jcs215152 (2018).
Gao, H., Liu, C. & Li, W. Assessing autophagy in the Leydig cells. Methods Mol. Biol. 1854, 71–85 (2019).
Roos, W. P., Thomas, A. D. & Kaina, B. DNA damage and the balance between survival and death in cancer biology. Nat. Rev. Cancer 16, 20–33 (2016).
Doherty, J. & Baehrecke, E. H. Life, death and autophagy. Nat. Cell Biol. 20, 1110–1117 (2018).
Gomez, Diaz C. & Ikeda, F. Roles of ubiquitin in autophagy and cell death. Semin. Cell Dev. Biol. S1084–9521, 30036–3 (2018).
Guo, C. et al. Amorphous silica nanoparticles trigger vascular endothelial cell injury through apoptosis and autophagy via reactive oxygen species-mediated MAPK/Bcl-2 and PI3K/Akt/mTOR signaling. Int. J. Nanomed. 11, 5257–5276 (2016).
Abais, J. M., **a, M., Zhang, Y., Boini, K. M. & Li, P. L. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid. Redox Signal. 22, 1111–1129 (2015).
Yang, J. et al. Hemorrhagic shock primes for lung vascular endothelial cell pyroptosis: role in pulmonary inflammation following LPS. Cell Death Dis. 7, e2363 (2016).
Liu, Q., Zhang, D., Hu, D., Zhou, X. & Zhou, Y. The role of mitochondria in NLRP3 inflammasome activation. Mol. Immunol. 103, 115–124 (2018).
Slowik, A., Lammerding, L., Zendedel, A., Habib, P. & Beyer, C. Impact of steroid hormones E2 and P on the NLRP3/ASC/Casp1 axis in primary mouse astroglia and BV-2 cells after in vitro hypoxia. J. Steroid Biochem. Mol. Biol. 183, 18–26 (2018).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
Zhang, Z. et al. Caspase-11-mediated tubular epithelial pyroptosis underlies contrast-induced acute kidney injury. Cell Death Dis. 9, 983 (2018).
Brentnall, M., Rodriguez-Menocal, L., Guevara, R. LDe., Cepero, E. & Boise, L. H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 14, 32 (2013).
Kim, K. H. & Lee, M. S. Autophagy—a key player in cellular and body metabolism. Nat. Rev. Endocrinol. 10, 322–337 (2014).
Kaminskyy, V. O. & Zhivotovsky, B. Free radicals in cross talk between autophagy and apoptosis. Antioxid. Redox Signal. 21, 86–102 (2014).
Sifuentes-Franco, S. et al. Oxidative stress, apoptosis, and mitochondrial function in diabetic nephropathy. Int. J. Endocrinol. 2018, 1875870 (2018).
Marino, G., Niso-Santano, M., Baehrecke, E. H. & Kroemer, G. Self-consumption: the interplay of autophagy and apoptosis. Nat. Revi. Mol. Cell Biol. 15, 81–94 (2014).
Parzych, K. R. & Klionsky, D. J. An overview of autophagy: morphology, mechanism, and regulation. Antioxid. Redox Signal. 20, 460–473 (2014).
Wong, W. T. et al. Repositioning of the beta-blocker carvedilol as a novel autophagy inducer that inhibits the NLRP3 inflammasome. Front. Immunol. 9, 1920 (2018).
Hu, Q. et al. The emerging role of stimulator of interferons genes signaling in sepsis: inflammation, autophagy and cell death. Acta physiol. 225, e13194 (2019).
Oka, S. et al. Role of heat shock factor 1 in conserving cholesterol transportation in Leydig cell steroidogenesis via steroidogenic acute regulatory protein. Endocrinology 158, 2648–2658 (2017).
Han, A. et al. ROS generation and MAPKs activation contribute to the Ni-induced testosterone synthesis disturbance in rat Leydig cells. Toxicol. Lett. 290, 36–45 (2018).
Gao, F. et al. Autophagy regulates testosterone synthesis by facilitating cholesterol uptake in Leydig cells. J. Cell Biol. 217, 2103–2119 (2018).
Li, W. R. et al. Autophagic deficiency is related to steroidogenic decline in aged rat Leydig cells. Asian J. Androl. 13, 881–888 (2011).
Li, G. H. et al. Ox-Lp(a) transiently induces HUVEC autophagy via an ROS-dependent PAPR-1-LKB1-AMPK-mTOR pathway. Atherosclerosis 243, 223–235 (2015).
Fan, X. et al. Berberine alleviates ox-LDL induced inflammatory factors by up-regulation of autophagy via AMPK/mTOR signaling pathway. J. Transl. Med. 13, 92 (2015).
Liu, S., Sun, Y. & Li, Z. Resveratrol protects Leydig cells from nicotine-induced oxidative damage through enhanced autophagy. Clin. Exp. Pharmacol. Physiol. 45, 573–580 (2018).
Wu, H., Song, A., Hu, W. & Dai, M. The anti-atherosclerotic effect of paeonol against vascular smooth muscle cell proliferation by up-regulation of autophagy via the AMPK/mTOR signaling pathway. Front. Pharmacol. 8, 948 (2017).
Jiang, X. et al. Effects of treatment with astragalus membranaceus on function of rat leydig cells. BMC Complement. Altern. Med. 15, 261 (2015).
Acknowledgements
This study was supported by the National Natural Science Foundation of China, Bei**g, China (Grant nos: 81501921, 81401190, 81871110, 81602241, 81471449 and 81671449), Hunan Natural Science Foundation, Hunan, China (Grant no: 2019JJ40269), Health and Family Planning Research Project of Hunan Province, Changsha, China (Grant no: B2017051), Science and Technology Project of Wuhan, China (Grant no: 2016060101010045), Social Development Foundation of Zhenjiang, Zhenjiang, China (Grant no: SH2016031), Guangdong Province Natural Science Foundation, Guangzhou, China (Grant no: 2015A030313141), Guangdong Province Science and Technology Project, Guangzhou, China (Grant nos: 2016B030230001 and 2016A040403113) and Key Scientific and Technological Program of Guangzhou City, Guangzhou, China (Grant no: 201604020189).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by G. M. Fimia
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, My., Zhu, Xl., Zhao, Bx. et al. Adrenomedullin alleviates the pyroptosis of Leydig cells by promoting autophagy via the ROS–AMPK–mTOR axis. Cell Death Dis 10, 489 (2019). https://doi.org/10.1038/s41419-019-1728-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-019-1728-5
- Springer Nature Limited
This article is cited by
-
Activation of SIRT1/Nrf2/HO-1 and Beclin-1/AMPK/mTOR autophagy pathways by eprosartan ameliorates testicular dysfunction induced by testicular torsion in rats
Scientific Reports (2024)
-
The roles of autophagy, ferroptosis and pyroptosis in the anti-ovarian cancer mechanism of harmine and their crosstalk
Scientific Reports (2024)
-
FoxG1 as a Potential Therapeutic Target for Alzheimer’s Disease: Modulating NLRP3 Inflammasome via AMPK/mTOR Autophagy Pathway
Cellular and Molecular Neurobiology (2024)
-
Thymoquinone effects on autophagy, apoptosis, and oxidative stress in cisplatin-induced testicular damage in mice
Journal of Assisted Reproduction and Genetics (2024)
-
Immune Response Gene-1 [IRG1]/itaconate protect against multi-organ injury via inhibiting gasdermin D-mediated pyroptosis and inflammatory response
Inflammopharmacology (2024)