Abstract
Pulmonary hypertension (PH) is a progressive fatal disease with no cure. Canagliflozin (CANA), a novel medication for diabetes, has been found to have remarkable cardiovascular benefits. However, few studies have addressed the effect and pharmacological mechanism of CANA in the treatment of PH. Therefore, our study aimed to investigate the effect and pharmacological mechanism of CANA in treating PH. First, CANA suppressed increased pulmonary artery pressure, right ventricular hypertrophy, and vascular remodeling in both mouse and rat PH models. Network pharmacology, transcriptomics, and biological results suggested that CANA could ameliorate PH by suppressing excessive oxidative stress and pulmonary artery smooth muscle cell proliferation partially through the activation of PPARγ. Further studies demonstrated that CANA inhibited phosphorylation of PPARγ at Ser225 (a novel serine phosphorylation site in PPARγ), thereby promoting the nuclear translocation of PPARγ and increasing its ability to resist oxidative stress and proliferation. Taken together, our study not only highlighted the potential pharmacological effect of CANA on PH but also revealed that CANA-induced inhibition of PPARγ Ser225 phosphorylation increases its capacity to counteract oxidative stress and inhibits proliferation. These findings may stimulate further research and encourage future clinical trials exploring the therapeutic potential of CANA in PH treatment.
Similar content being viewed by others
Introduction
Pulmonary hypertension (PH) is a severe and incurable disease that seriously affects the quality of life and life expectancy of patients [1]. Although targeted drugs have been developed in recent years, they still have limitations with regard to efficacy and safety [2, 3]. Therefore, further exploration of novel medications for treating PH to improve treatment effectiveness and alleviate patient burden is urgently needed.
The primary pathological alterations in PH involve the impairment and destruction of pulmonary artery endothelial cells (PAECs), abnormal growth of pulmonary artery smooth muscle cells (PASMCs), and the accumulation of extravascular collagen [4, 5]. These pathological processes are also accompanied by the oxidative stress response of pulmonary artery cells [6]. Oxidative stress is primarily characterized by an abundance of reactive oxygen species (ROS) within cells. ROS are also critical signaling molecules for vascular cell proliferation [7, 8]. Vitry et al. showed that PH-PASMCs could exert hyperproliferative and antiapoptotic effects by hijacking persistent oxidative stress [9]. Hennigs et al. reported a PPARγ- and P53-mediated mechanism for vascular protection in PH-PAECs in response to DNA damage and oxidative stress [10]. Yeligar et al. reported that depletion of PPARγ in human PASMCs increased mitochondria-derived ROS production and disrupted mitochondrial bioenergetics [11]. These results suggest that PPARγ-driven inhibition of oxidative stress is critical for vascular remodeling in PH. Consequently, finding an effective drug targeting these pathological hallmarks is of interest for the management of PH.
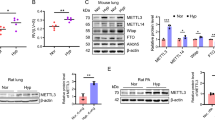
Canagliflozin (CANA) is an effective hypoglycaemic drug that is approved in the USA for type II diabetes treatment. Nevertheless, in recent years, an increasing number of studies have revealed its favorable protective effects on the cardiovascular system. For example, numerous studies have indicated that CANA might impact vasorelaxation in arterioles from human visceral adipose tissue [12], hinder the proliferation of vascular smooth muscle cells in blood vessels and decrease the progression of atherosclerosis [13, 14]. Furthermore, a study revealed that CANA can substantially reduce the occurrence of major cardiovascular incidents in individuals with diabetes and chronic kidney disease [15] while also notably diminishing the cardiovascular hazard for patients suffering from heart failure [16]. The protective effect of CANA on the cardiovascular system may be attributed to its ability to inhibit cell proliferation [17], fibrosis [18], and oxidative stress [19]. However, the therapeutic effect and underlying mechanisms of CANA on PH remain unclear, and a better understanding of these effects could provide us with novel insights into PH treatment.
Here, we combined network pharmacology, transcriptomics, and biological methods to evaluate the promising protective effect of CANA against PH. The pharmacological protective effect of CANA on PH was investigated in three common PH rodent models and a hypoxia-induced PH cell model in this study. Network pharmacology and transcriptomics methods revealed that PPARγ was the target and signaling molecule for the anti-PH effect of CANA, which suppressed oxidative stress and excessive proliferation of PASMCs. Subsequent validation experiments were conducted in vivo and in vitro to assess the anti-PH effects of CANA through PPARγ. Moreover, we showed that CANA targeted PPARγ and inhibited PPARγ S225 phosphorylation, thus exerting its antiproliferative and antioxidative effects on stress. Notably, this is the first time that CANA has been investigated and validated in the field of PH via multiple bioinformatics and biological methods. Figure 1 displays a schematic illustration of the investigation.
The animation graph condensed the study method and proposed mechanism demonstrated in this work. Here, we integrate network pharmacology, transcriptomics, molecular docking, and experimental verification to uncover the role and mechanism of CANA on PH. 5 animal models are used as shown in the image. We report that CANA inhibits PPARγ Ser225 phosphorylation, promoting nuclear translocation of PPARγ, and enhancing oxidative stress-related gene translation, thus inhibiting ros accumulation, proliferation, and migration of rPASMCs.
Materials and methods
Animal models
This study involved the creation of various animal models, including a mouse model of hypoxia-induced (Hx) PH, mouse and rat models of severe PH induced by Sugen 5416-hypoxia (Su/Hx), a model for rescuing established PH and a rat model of PH induced by monocrotaline (MCT) [4, 5, 20]. All mice and rats were purchased from Weitong Lihua Limited Company (Bei**g, China). Before the experiments, all animals were allowed to acclimate to the pathogen-free animal habitat for a minimum of 1 week. Additionally, an ample supply of food (consisting of 24% protein, 15% fat, and 61% carbohydrates, sourced from Jiangsu ** rat brain: effect of metal mixture and troglitazone in astrocytes. Cell Death Dis. 2014;5:e1033." href="/article/10.1038/s41401-024-01286-9#ref-CR56" id="ref-link-section-d82699633e2139">56]. PPARγ activation also suppressed rPASMC proliferation and promoted eNOS expression, increasing the release of NO from endothelial cells [57, 58]. The results of this study indicate that CANA significantly increases the expression of PPARγ both in vivo and in vitro. Based on the evidence above, we presumed that PPARγ plays a key role in the anti-PH effects of CANA. The other 4 core genes (eNOS, iNOS, ACE, MMP9) were not initially considered for the following reasons: (1) our transcriptome data indicated significant enrichment of the PPAR signaling pathway after CANA treatment, highlighting the importance of PPARγ in the anti-PH effect of CANA; (2) PPARγ showed a strong association with oxidative stress, which was also significantly enriched in the transcriptome and network pharmacology analysis; and (3) our previous studies emphasized the crucial role of PPARγ in PH pathology in both rPASMCs and endothelial cells, while eNOS, iNOS, and ACE were primarily associated with endothelial cells [45, 46, 59], and MMP9 was related to fibroblasts [60, 61]. Since PAMSCs are key cells involved in vascular remodeling, our study focused specifically on the role of rPASMCs in the pathogenesis of PH. However, further research is needed to determine whether CANA affects lung endothelial cells through PPARγ.
CANA, dapagliflozin, and empagliflozin are classified as inhibitors of sodium-glucose cotransporter 2 (SGLT2), where SGLT2 accounts for the majority of renal glucose reabsorption in the kidneys [62]. Various studies have shown that CANA has a weaker inhibitory effect on SGLT2 than dapagliflozin and empagliflozin, suggesting that CANA might exhibit more specific and potent inhibition of other proteins [63, 64]. Similar to scholarly research indicating the absence of SGLT2 in any blood vessel other than the kidney, our investigation using the THPA database revealed a low level of SGLT2 protein in rPASMCs. Hence, we did not examine the effect of CANA on SGLT2 in our models. Our findings confirmed that CANA can directly bind to PPARγ, thereby hindering its phosphorylation. This discovery introduces a novel perspective on CANA. Notably, in addition to SGLT2 inhibitors regulating blood sugar levels, SGLT2 inhibitors such as CANA are being increasingly used due to their supplementary advantages for cardiovascular health [65, 66].
In PH associated with heart failure with preserved ejection fraction (HFpEF), Taijyu et al. [67] reported that empagliflozin decreased mitochondrial ROS and increased pulmonary arterial remodeling while exercising. ZSF-1 obese rats were used as a rodent model for HFpEF and PH, either with or without Sugen treatment. These models exhibit numerous characteristics similar to those of type II diabetes in humans [68]. According to the authors, in this particular situation, empagliflozin ameliorated metabolic syndrome, thereby inhibiting the initial development of mitochondrial ROS [67]. We utilized hypoxia-related pulmonary hypertension (PH) models from the third category, which lacked a distinct metabolic profile (such as diabetes) similar to that of obese ZSF-1 rats. Hence, these metabolic features were not evaluated in this study. Similar to the role of empagliflozin in HFpEF, CANA also ameliorated oxidative stress and pulmonary artery remodeling in hypoxia-related PH in our study. However, we highlight the role of PPARγ in the anti-PH effects of CANA. Indeed, PPARγ and the PPAR coactivator PGC1-α are thought to be closely related to mitochondrial oxidative stress. In addition, there is no direct evidence that CANA modulates oxidative stress via SLGT2 inhibition.
In short, different SGLT2 inhibitors might have different effects on different clinical types of PH. Hess et al. [69] found that empagliflozin attenuated MCT-induced PH, but its molecular mechanism of action was nonspecific. Wu et al. [70] showed that dapagliflozin has no protective effect on PH models. Recently, Tang et al. [71] showed that CANA had a protective effect on hypoxia-induced PH, which is consistent with our conclusions. However, our exploration of the role and mechanism of CANA in PH is undoubtedly multiangled, comprehensive, and in depth.
PPARγ activation has been found to prevent PH progression. According to our previous investigation, rosiglitazone, a conventional activator of PPARγ, can effectively alleviate pulmonary vascular remodeling and right ventricular remodeling in a mouse model of hypoxia-induced PH [28]. Legchenko et al. reported that the PPARγ agonist pioglitazone reversed PH and prevented right heart failure [52]. Pioglitazone was also found to prevent the development of MCT-induced PH in rats [72]. However, several clinical trials with these PPARγ agonists, such as rosiglitazone, have demonstrated that they may increase heart failure. This finding can be explained by their side effects, with a focus on mechanisms such as edema and sodium retention. The clinical use of full-spectrum PPARγ agonists, such as rosiglitazone, is limited due to the cardiovascular risks associated with their long-term use. In the treatment of diabetes, one strategy is the development of selective PPARγ modulators, SPPARMs, to preserve the expression level of PPARγ in vivo and regulate the transcription of therapeutic-related target genes while avoiding the regulation of target genes linked to adverse reactions [73]. Our data provide some support for the use of CANA as a selective PPARγ modulator, which holds promise for the treatment of PH. However, more experiments are needed to support this hypothesis.
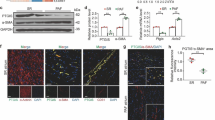
Protein functions can be regulated by post-translational modifications, most commonly phosphorylation [74]. Depending on the cell type and stimulation, the site of phosphorylation of PPARγ and its biological effects are completely different [75, 76]. The phosphorylation sites that have been identified in PPARγ include Ser112 and Ser273. PPARγ Ser112 phosphorylation typically leads to reduced PPARγ activity [77], while PPARγ phosphorylation at Ser273 occurs mainly in adipose tissue [78]. Phosphorylation of PPARγ Ser225 (S225) has not been reported. Our results suggested that PPARγ S225 expression was altered in the pathogenesis of PH, which inhibited PPARγ transfer into the nucleus and thus reduced PPARγ transcriptional activity. However, CANA directly interacts with PPARγ, inhibiting PPARγ S225 phosphorylation and promoting PPARγ activity, resulting in excellent antioxidant stress effects. However, a larger in vivo dataset would result in further insight into this issue.
The findings of this research suggest that CANA has potential for use as a treatment for PH. This drug improved the progression of PH by reducing damage caused by oxidative stress and inhibiting the proliferation of smooth muscle cells in the pulmonary artery. This effect is partially achieved by activating PPARγ. Mechanistically, CANA inhibits PPARγ S225 phosphorylation and promotes PPARγ function. Taken together, the results of this study may help to elucidate the molecular process underlying the emergence and progression of PH while also revealing a novel avenue for the practical utilization of CANA.
Data availability
Data will be made available upon request.
References
Cruz-Utrilla A, Pérez-Olivares C, Martínez-Meñaca A, López-Meseguer M, Escribano-Subias P. Phenotypes of idiopathic pulmonary arterial hypertension. Lancet Respir Med. 2022;10:e87.
Saggar R, Abtin F, Channick R. Inhaled treprostinil in Group 3 pulmonary hypertension. N Engl J Med. 2021;384:1870.
Humbert M, McLaughlin V, Gibbs JSR, Gomberg-Maitland M, Hoeper MM, Preston IR, et al. Sotatercept for the treatment of pulmonary arterial hypertension. N Engl J Med. 2021;384:1204–15.
Li X, Zhang Y, Su L, Cai L, Zhang C, Zhang J, et al. FGF21 alleviates pulmonary hypertension by inhibiting mTORC1/EIF4EBP1 pathway via H19. J Cell Mol Med. 2022;26:3005–21.
Su L, Li X, Mao X, Xu T, Zhang Y, Li S, et al. Circ-Ntrk2 acts as a miR-296-5p sponge to activate the TGF-β1/p38 MAPK pathway and promote pulmonary hypertension and vascular remodelling. Respir Res. 2023;24:78.
Badran A, Nasser SA, Mesmar J, El-Yazbi AF, Bitto A, Fardoun MM, et al. Reactive oxygen species: modulators of phenotypic switch of vascular smooth muscle cells. Int J Mol Sci. 2020;21:8764.
Diebold L, Chandel NS. Mitochondrial ROS regulation of proliferating cells. Free Radic Biol Med. 2016;100:86–93.
Byon CH, Heath JM, Chen Y. Redox signaling in cardiovascular pathophysiology: a focus on hydrogen peroxide and vascular smooth muscle cells. Redox Biol. 2016;9:244–53.
Vitry G, Paulin R, Grobs Y, Lampron MC, Shimauchi T, Lemay SE, et al. Oxidized DNA precursors cleanup by NUDT1 contributes to vascular remodeling in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2021;203:614–27.
Hennigs JK, Cao A, Li CG, Shi M, Mienert J, Miyagawa K, et al. PPARγ-p53-mediated vasculoregenerative program to reverse pulmonary hypertension. Circ Res. 2021;128:401–18.
Yeligar SM, Kang BY, Bijli KM, Kleinhenz JM, Murphy TC, Torres G, et al. PPARγ regulates mitochondrial structure and function and human pulmonary artery smooth muscle cell proliferation. Am J Respir Cell Mol Biol. 2018;58:648–57.
De Stefano A, Tesauro M, Di Daniele N, Vizioli G, Schinzari F, Cardillo C. Mechanisms of SGLT2 (sodium-glucose transporter type 2) inhibition-induced relaxation in arteries from human visceral adipose tissue. Hypertension. 2021;77:729–38.
Behnammanesh G, Durante GL, Khanna YP, Peyton KJ, Durante W. Canagliflozin inhibits vascular smooth muscle cell proliferation and migration: role of heme oxygenase-1. Redox Biol. 2020;32:101527.
Nasiri-Ansari Ν, Dimitriadis GK, Agrogiannis G, Perrea D, Kostakis ID, Kaltsas G, et al. Canagliflozin attenuates the progression of atherosclerosis and inflammation process in APOE knockout mice. Cardiovasc Diabetol. 2018;17:106.
Neal B, Perkovic V, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:2099.
Seferović PM, Coats AJS, Ponikowski P, Filippatos G, Huelsmann M, Jhund PS, et al. European Society of Cardiology/Heart Failure Association position paper on the role and safety of new glucose-lowering drugs in patients with heart failure. Eur J Heart Fail. 2020;22:196–213.
Behnammanesh G, Durante ZE, Peyton KJ, Martinez-Lemus LA, Brown SM, Bender SB, et al. Canagliflozin inhibits human endothelial cell proliferation and tube formation. Front Pharmacol. 2019;10:362.
Heerspink HJL, Perco P, Mulder S, Leierer J, Hansen MK, Heinzel A, et al. Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia. 2019;62:1154–66.
Hasan R, Lasker S, Hasan A, Zerin F, Zamila M, Chowdhury FI, et al. Canagliflozin attenuates isoprenaline-induced cardiac oxidative stress by stimulating multiple antioxidant and anti-inflammatory signaling pathways. Sci Rep. 2020;10:14459.
Liu J, Cai G, Li M, Fan S, Yao B, ** W, et al. Fibroblast growth factor 21 attenuates hypoxia-induced pulmonary hypertension by upregulating PPARγ expression and suppressing inflammatory cytokine levels. Biochem Biophys Res Commun. 2018;504:478–84.
Huang X, Mao W, Zhang T, Wang M, Wang X, Li Y, et al. Baicalin promotes apoptosis and inhibits proliferation and migration of hypoxia-induced pulmonary artery smooth muscle cells by up-regulating A2a receptor via the SDF-1/CXCR4 signaling pathway. BMC Complement Altern Med. 2018;18:330.
Zheng D, Zhu Y, Shen Y, **ao S, Yang L, **ang Y, et al. Cynaropicrin shows antitumor progression potential in colorectal cancer through mediation of the LIFR/STATs axis. Front Cell Dev Biol. 2020;8:605184.
Daina A, Michielin O, Zoete V. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019;47:W357–w64.
Wang X, Shen Y, Wang S, Li S, Zhang W, Liu X, et al. PharmMapper 2017 update: a web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017;45:W356–w60.
Yu H, Chen J, Xu X, Li Y, Zhao H, Fang Y, et al. A systematic prediction of multiple drug-target interactions from chemical, genomic, and pharmacological data. PLoS One. 2012;7:e37608.
Wang X, Pan C, Gong J, Liu X, Li H. Enhancing the enrichment of pharmacophore-based target prediction for the polypharmacological profiles of drugs. J Chem Inf Model. 2016;56:1175–83.
Yao D, He Q, Sun J, Cai L, Wei J, Cai G, et al. FGF21 attenuates hypoxia‑induced dysfunction and inflammation in HPAECs via the microRNA‑27b‑mediated PPARγ pathway. Int J Mol Med. 2021;47:116.
Cai G, Liu J, Wang M, Su L, Cai M, Huang K, et al. Mutual promotion of FGF21 and PPARγ attenuates hypoxia-induced pulmonary hypertension. Exp Biol Med. 2019;244:252–61.
Lee C. Collaborative power of Nrf2 and PPARγ activators against metabolic and drug-induced oxidative injury. Oxid Med Cell Longev. 2017;2017:1378175.
Liu YD, Yu SL, Wang R, Liu JN, ** YS, Li YF, et al. Rosiglitazone suppresses calcium oxalate crystal binding and oxalate-induced oxidative stress in renal epithelial cells by promoting PPAR-γ activation and subsequent regulation of TGF-β1 and HGF expression. Oxid Med Cell Longev. 2019;2019:4826525.
Tonelli C, Chio IIC, Tuveson DA. Transcriptional regulation by Nrf2. Antioxid Redox Signal. 2018;29:1727–45.
Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci. 2016;73:3221–47.
Tseng V, Sutliff RL, Hart CM. Redox biology of peroxisome proliferator-activated receptor-γ in pulmonary hypertension. Antioxid Redox Signal. 2019;31:874–97.
Rius-Pérez S, Torres-Cuevas I, Millán I, Ortega ÁL, Pérez S. PGC-1α, inflammation, and oxidative stress: an integrative view in metabolism. Oxid Med Cell Longev. 2020;2020:1452696.
Glorieux C, Zamocky M, Sandoval JM, Verrax J, Calderon PB. Regulation of catalase expression in healthy and cancerous cells. Free Radic Biol Med. 2015;87:84–97.
Wang Y, Branicky R, Noë A, Hekimi S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol. 2018;217:1915–28.
Zhao N, Kamijo K, Fox PD, Oda H, Morisaki T, Sato Y, et al. A genetically encoded probe for imaging nascent and mature HA-tagged proteins in vivo. Nat Commun. 2019;10:2947.
Song T, Yang Y, Wei H, **e X, Lu J, Zeng Q, et al. Zfp217 mediates m6A mRNA methylation to orchestrate transcriptional and post-transcriptional regulation to promote adipogenic differentiation. Nucleic Acids Res. 2019;47:6130–44.
Hall JA, Ramachandran D, Roh HC, DiSpirito JR, Belchior T, Zushin PH, et al. Obesity-linked PPARγ S273 phosphorylation promotes insulin resistance through growth differentiation factor 3. Cell Metab. 2020;32:665–75.e6.
Humbert M, Guignabert C, Bonnet S, Dorfmüller P, Klinger JR, Nicolls MR, et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J. 2019;53:1801887.
Shimizu T, Higashijima Y, Kanki Y, Nakaki R, Kawamura T, Urade Y, et al. PERK inhibition attenuates vascular remodeling in pulmonary arterial hypertension caused by BMPR2 mutation. Sci Signal. 2021;14:eabb3616.
Liu Z, Ma X, Ilyas I, Zheng X, Luo S, Little PJ, et al. Impact of sodium glucose cotransporter 2 (SGLT2) inhibitors on atherosclerosis: from pharmacology to pre-clinical and clinical therapeutics. Theranostics. 2021;11:4502–15.
Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem. 2009;78:959–91.
Zhao S, Iyengar R. Systems pharmacology: network analysis to identify multiscale mechanisms of drug action. Annu Rev Pharmacol Toxicol. 2012;52:505–21.
Fysikopoulos A, Seimetz M, Hadzic S, Knoepp F, Wu CY, Malkmus K, et al. Amelioration of elastase-induced lung emphysema and reversal of pulmonary hypertension by pharmacological iNOS inhibition in mice. Br J Pharmacol. 2021;178:152–71.
Hua C, Zhao J, Wang H, Chen F, Meng H, Chen L, et al. Apple polyphenol relieves hypoxia-induced pulmonary arterial hypertension via pulmonary endothelium protection and smooth muscle relaxation: In vivo and in vitro studies. Biomed Pharmacother. 2018;107:937–44.
Yan S, Wang Y, Liu P, Chen A, Chen M, Yao D, et al. Baicalin attenuates hypoxia-induced pulmonary arterial hypertension to improve hypoxic cor pulmonale by reducing the activity of the p38 MAPK signaling pathway and MMP-9. Evid Based Complement Altern Med. 2016;2016:2546402.
Zhang R, Wu Y, Zhao M, Liu C, Zhou L, Shen S, et al. Role of HIF-1alpha in the regulation ACE and ACE2 expression in hypoxic human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2009;297:L631–40.
Mukohda M, Ozaki H. Anti-inflammatory mechanisms of the vascular smooth muscle PPARγ. J Smooth Muscle Res. 2021;57:1–7.
Li HY, Yang M, Li Z, Meng Z. Curcumin inhibits angiotensin II-induced inflammation and proliferation of rat vascular smooth muscle cells by elevating PPAR-γ activity and reducing oxidative stress. Int J Mol Med. 2017;39:1307–16.
Ding G, Fu M, Qin Q, Lewis W, Kim HW, Fukai T, et al. Cardiac peroxisome proliferator-activated receptor gamma is essential in protecting cardiomyocytes from oxidative damage. Cardiovasc Res. 2007;76:269–79.
Okuno Y, Matsuda M, Miyata Y, Fukuhara A, Komuro R, Shimabukuro M, et al. Human catalase gene is regulated by peroxisome proliferator-activated receptor-gamma through a response element distinct from that of mouse. Endocr J. 2010;57:303–9.
Chen T, ** X, Crawford BH, Cheng H, Saafir TB, Wagner MB, et al. Cardioprotection from oxidative stress in the newborn heart by activation of PPARγ is mediated by catalase. Free Radic Biol Med. 2012;53:208–15.
Zhou Y, Zhang MJ, Li BH, Chen L, Pi Y, Yin YW, et al. PPARγ inhibits VSMC proliferation and migration via attenuating oxidative stress through upregulating UCP2. PLoS One. 2016;11:e0154720.
Garg M, Johri S, Sagar S, Mundhada A, Agrawal A, Ray P, et al. Cardiolipin-mediated PPARγ S112 phosphorylation impairs IL-10 production and inflammation resolution during bacterial pneumonia. Cell Rep. 2021;34:108736.
Rai A, Tripathi S, Kushwaha R, Singh P, Srivastava P, Sanyal S, et al. CDK5-induced p-PPARγ(Ser 112) downregulates GFAP via PPREs in develo** rat brain: effect of metal mixture and troglitazone in astrocytes. Cell Death Dis. 2014;5:e1033.
Song J, Li C, Lv Y, Zhang Y, Amakye WK, Mao L. DHA increases adiponectin expression more effectively than EPA at relative low concentrations by regulating PPARγ and its phosphorylation at Ser273 in 3T3-L1 adipocytes. Nutr Metab. 2017;14:52.
Huang XY, Song LL, Wang XH, Zhu XY, Tong SL, Chen ZL, et al. Canagliflozin alleviates pulmonary hypertension partially by inhibition of PPARγ S225 phosphorylation and PPARγ-mediated suppression of oxidative stress. Research Square. 2023.
Wang M, Su L, Sun J, Cai L, Li X, Zhu X, et al. FGF21 attenuates pulmonary arterial hypertension via downregulation of miR-130, which targets PPARγ. J Cell Mol Med. 2022;26:1034–49.
Legchenko E, Chouvarine P, Borchert P, Fernandez-Gonzalez A, Snay E, Meier M, et al. PPARγ agonist pioglitazone reverses pulmonary hypertension and prevents right heart failure via fatty acid oxidation. Sci Transl Med. 2018;10:eaao0303.
Idris-Khodja N, Ouerd S, Trindade M, Gornitsky J, Rehman A, Barhoumi T, et al. Vascular smooth muscle cell peroxisome proliferator-activated receptor γ protects against endothelin-1-induced oxidative stress and inflammation. J Hypertens. 2017;35:1390–401.
Maccallini C, Mollica A, Amoroso R. The positive regulation of eNOS signaling by PPAR agonists in cardiovascular diseases. Am J Cardiovasc Drugs. 2017;17:273–81.
Jiang S, Chen G, Yang Z, Wang D, Lu Y, Zhu L, et al. Testosterone attenuates hypoxia-induced hypertension by affecting NRF1-mediated transcriptional regulation of ET-1 and ACE. Hypertens Res. 2021;44:1395–405.
Zheng Z, Li J, Cui Y, Wang W, Zhang M, Zhang Y, et al. IRAK-M regulates proliferative and invasive phenotypes of lung fibroblasts. Inflammation. 2023;46:763–78.
Ortega-Paz L, Cristóbal H, Ortiz-Perez JT, García de Frutos P, Mendieta G, Sandoval E, et al. Direct actions of dapagliflozin and interactions with LCZ696 and spironolactone on cardiac fibroblasts of patients with heart failure and reduced ejection fraction. ESC Heart Fail. 2023;10:453–64.
Chao EC, Henry RR. SGLT2 inhibition—a novel strategy for diabetes treatment. Nat Rev Drug Discov. 2010;9:551–9.
Lin Y, Nan J, Shen J, Lv X, Chen X, Lu X, et al. Canagliflozin impairs blood reperfusion of ischaemic lower limb partially by inhibiting the retention and paracrine function of bone marrow derived mesenchymal stem cells. EBioMedicine. 2020;52:102637.
Hung MH, Chen YL, Chen LJ, Chu PY, Hsieh FS, Tsai MH, et al. Canagliflozin inhibits growth of hepatocellular carcinoma via blocking glucose-influx-induced β-catenin activation. Cell Death Dis. 2019;10:420.
Sabe SA, Xu CM, Sabra M, Harris DD, Malhotra A, Aboulgheit A, et al. Canagliflozin improves myocardial perfusion, fibrosis, and function in a swine model of chronic myocardial ischemia. J Am Heart Assoc. 2023;12:e028623.
Nassif ME, Qintar M, Windsor SL, Jermyn R, Shavelle DM, Tang F, et al. Empagliflozin effects on pulmonary artery pressure in patients with heart failure: results from the EMBRACE-HF trial. Circulation. 2021;143:1673–86.
Satoh T, Wang L, Espinosa-Diez C, Wang B, Hahn SA, Noda K, et al. Metabolic syndrome mediates ROS-miR-193b-NFYA-dependent downregulation of soluble guanylate cyclase and contributes to exercise-induced pulmonary hypertension in heart failure with preserved ejection fraction. Circulation. 2021;144:615–37.
Su Z, Widomski D, Ma J, Namovic M, Nikkel A, Leys L, et al. Longitudinal changes in measured glomerular filtration rate, renal fibrosis and biomarkers in a rat model of type 2 diabetic nephropathy. Am J Nephrol. 2016;44:339–53.
Chowdhury B, Luu AZ, Luu VZ, Kabir MG, Pan Y, Teoh H, et al. The SGLT2 inhibitor empagliflozin reduces mortality and prevents progression in experimental pulmonary hypertension. Biochem Biophys Res Commun. 2020;524:50–6.
Li H, Zhang Y, Wang S, Yue Y, Liu Q, Huang S, et al. Dapagliflozin has no protective effect on experimental pulmonary arterial hypertension and pulmonary trunk banding rat models. Front Pharmacol. 2021;12:756226.
Tang L, Cai Q, Wang X, Li X, Li X, Chen L, et al. Canagliflozin ameliorates hypobaric hypoxia-induced pulmonary arterial hypertension by inhibiting pulmonary arterial smooth muscle cell proliferation. Clin Exp Hypertens. 2023;45:2278205.
Hussain T, Chai L, Wang Y, Zhang Q, Wang J, Shi W, et al. Activation of PPAR-γ prevents TERT-mediated pulmonary vascular remodeling in MCT-induced pulmonary hypertension. Heliyon. 2023;9:e14173.
Huan Y, Pan X, Peng J, Jia C, Sun S, Bai G, et al. A novel specific peroxisome proliferator-activated receptor γ (PPARγ) modulator YR4-42 ameliorates hyperglycaemia and dyslipidaemia and hepatic steatosis in diet-induced obese mice. Diabetes Obes Metab. 2019;21:2553–63.
Newcombe EA, Delaforge E, Hartmann-Petersen R, Skriver K, Kragelund BB. How phosphorylation impacts intrinsically disordered proteins and their function. Essays Biochem. 2022;66:901–13.
Stechschulte LA, Czernik PJ, Rotter ZC, Tausif FN, Corzo CA, Marciano DP, et al. PPARG post-translational modifications regulate bone formation and bone resorption. EBioMedicine. 2016;10:174–84.
Acknowledgements
We acknowledge the support from the First Affiliated Hospital of Wenzhou Medical University Medical Research Center. We express gratitude to Cifeng Cai from Hangzhou Cosmos Wisdom for his technical support in experiment. This work was supported by the National Natural Science Foundation of China (82170061, 82241001) and Zhejiang Province Natural Fund (LQ24H010004). A preprint has previously been published [79].
Author information
Authors and Affiliations
Contributions
XCL, LXW, and XYH designed research; XCL, YYW, ZLC, JHZ, LLS, XHW, YHS, and LHS carried out animal experiments and analyzed the data. XCL, XYZ, SLT, ZYL, CZ, and CYZ performed the in vitro experiments, and collected and analyzed the data. XCL and CZ perform bioinformatic analysis. XCL, XYZ, and YYW wrote the first draft and all authors contributed to the review and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Xc., Zhu, Xy., Wang, Yy. et al. Canagliflozin alleviates pulmonary hypertension by activating PPARγ and inhibiting its S225 phosphorylation. Acta Pharmacol Sin (2024). https://doi.org/10.1038/s41401-024-01286-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41401-024-01286-9
- Springer Nature Singapore Pte Ltd.