Abstract
Objectives
Hepatic ischemia and hypoxia are accompanied by reduced bile flow, biliary sludge and cholestasis. Hepatobiliary transport systems, nuclear receptors and aquaporins were studied after hypoxia and reoxygenation in human hepatic cells.
Methods
Expression of Aquaporin 8 (AQP8), Aquaporin 9 (AQP9), Pregnane X receptor (PXR), Farnesoid X receptor (FXR), Organic anion transporting polypeptide 1 (OATP1), and the Multidrug resistance-associated protein 4 (MRP4) were investigated in induced pluripotent stem cells (iPSCs) derived hepatic cells and the immortalized hepatic line HepG2. HepG2 was subjected to combined oxygen and glucose deprivation for 4 h followed by reoxygenation.
Results
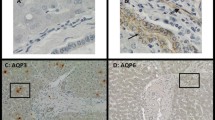
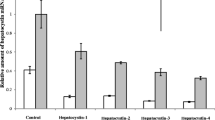
Expression of AQP8 and AQP9 increased during differentiation in iPSC-derived hepatic cells. Hypoxia did not alter mRNA levels of AQP8, but reoxygenation caused a marked increase in AQP8 mRNA expression. While expression of OATP1 had a transient increase during reoxygenation, MRP4 showed a delayed downregulation. Knock-down of FXR did not alter the expression of AQP8, AQP9, MRP4, or OATP1. Post-hypoxic protein levels of AQP8 were reduced after 68 h of reoxygenation compared to normoxic controls.
Conclusions
Post-transcriptional mechanisms rather than reduced transcription cause reduction in AQP8 protein concentration after hypoxia-reoxygenation in hepatic cells. Expression patterns differed between hepatobiliary transport systems during hypoxia and reoxygenation.
Impact
-
Expression of AQP8 and AQP9 increased during differentiation in induced pluripotent stem cells.
-
Expression of hepatobiliary transporters varies during hypoxia and reoxygenation.
-
Post-hypoxic protein levels of AQP8 were reduced after 68 h of reoxygenation.
-
Post-transcriptional mechanisms rather than reduced transcription cause reduction in AQP8 protein concentration after hypoxia-reoxygenation in hepatic cells.
-
Hypoxia and reoxygenation may affect aquaporins in hepatic cells and potentially affect bile composition.





Similar content being viewed by others
References
Fouassier, L. et al. Hypoxia-induced changes in the expression of rat hepatobiliary transporter genes. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G25–G35 (2007).
Keizman, D., Ish-Shalom, M. & Konikoff, F. M. The clinical significance of bile duct sludge: is it different from bile duct stones? Surg. Endosc. 21, 769–773 (2007).
Konno, H., Hardison, W. G. & Miyai, K. Reoxygenation injury following anoxic perfusion preferentially impairs bile acid-independent bile flow. Eur. Surg. Res. 23, 151–157 (1991).
Fu**o, T. et al. Hypoxia downregulates farnesoid X receptor via a hypoxia-inducible factor-independent but p38 mitogen-activated protein kinase-dependent pathway. FEBS J. 276, 1319–1332 (2009).
Schaffner, C. A. et al. The organic solute transporters alpha and beta are induced by hypoxia in human hepatocytes. Liver Int. 35, 1152–1161 (2015).
Larocca, M. C., Soria, L. R., Espelt, M. V., Lehmann, G. L. & Marinelli, R. A. Knockdown of hepatocyte aquaporin-8 by RNA interference induces defective bile canalicular water transport. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G93–G100 (2009).
Elkjaer, M. et al. Immunolocalization of AQP9 in liver, epididymis, testis, spleen, and brain. Biochem. Biophys. Res. Commun. 276, 1118–1128 (2000).
Bienert, G. P. et al. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 282, 1183–1192 (2007).
Ma, T., Yang, B. & Verkman, A. S. Cloning of a novel water and urea-permeable aquaporin from mouse expressed strongly in colon, placenta, liver, and heart. Biochem. Biophys. Res. Commun. 240, 324–328 (1997).
Vieceli Dalla Sega, F. et al. Specific aquaporins facilitate Nox-produced hydrogen peroxide transport through plasma membrane in leukaemia cells. Biochim. Biophys. Acta 1843, 806–814 (2014).
Marchissio, M. J., Francés, D. E., Carnovale, C. E. & Marinelli, R. A. Mitochondrial aquaporin-8 knockdown in human hepatoma HepG2 cells causes ROS-induced mitochondrial depolarization and loss of viability. Toxicol. Appl Pharm. 264, 246–254 (2012).
Huebert, R. C., Splinter, P. L., Garcia, F., Marinelli, R. A. & LaRusso, N. F. Expression and localization of aquaporin water channels in rat hepatocytes. Evidence for a role in canalicular bile secretion. J. Biol. Chem. 277, 22710–22717 (2002).
Carreras, F. I. et al. Defective hepatocyte aquaporin-8 expression and reduced canalicular membrane water permeability in estrogen-induced cholestasis. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G905–G912 (2007).
Lehmann, G. L., Carreras, F. I., Soria, L. R., Gradilone, S. A. & Marinelli, R. A. LPS induces the TNF-alpha-mediated downregulation of rat liver aquaporin-8: role in sepsis-associated cholestasis. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G567–G575 (2008).
Asai, Y. et al. Activation of the hypoxia inducible factor 1alpha subunit pathway in steatotic liver contributes to formation of cholesterol gallstones. Gastroenterol 152, 1521–1535.e8 (2017).
Karimi, S., Khatami, S. R., Azarpira, N., Galehdari, H. & Pakbaz, S. Investigate of AQP gene expression in the liver of mice after ischemia –reperfusion. Mol. Biol. Rep. 45, 1769–1774 (2018).
Setchell, K. D. et al. Bile acid concentrations in human and rat liver tissue and in hepatocyte nuclei. Gastroenterology 112, 226–235 (1997).
Fattinger, K. et al. The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin. Pharmacol. Ther. 69, 223–231 (2001).
Leslie, E. M., Watkins, P. B., Kim, R. B. & Brouwer, K. L. Differential inhibition of rat and human Na+-dependent taurocholate cotransporting polypeptide (NTCP/SLC10A1) by bosentan: a mechanism for species differences in hepatotoxicity. J. Pharmacol. Exp. Ther. 321, 1170–1178 (2007).
Pisal, R. V. et al. Directed reprogramming of comprehensively characterized dental pulp stem cells extracted from natal tooth. Sci. Rep. 8, 6168 (2018).
Siller, R., Greenhough, S., Naumovska, E. & Sullivan, G. J. Small-molecule-driven hepatocyte differentiation of human pluripotent stem cells. Stem Cell Rep. 4, 939–952 (2015).
Siller, R. et al. Development of a rapid screen for the endodermal differentiation potential of human pluripotent stem cell lines. Sci. Rep. 6, 37178 (2016).
Mathapati, S. et al. Small-molecule-directed hepatocyte-like cell differentiation of human pluripotent stem cells. Curr. Protoc. Stem. Cell. Biol. 38, 1G.6.1–1G.6.18 (2016).
Almaas, R., Saugstad, O. D., Pleasure, D. & Rootwelt, T. Effect of barbiturates on hydroxyl radicals, lipid peroxidation, and hypoxic cell death in human NT2-N neurons. Anesthesiol 92, 764–774 (2000).
Rootwelt, T. et al. Hypoxic cell death in human NT2-N neurons: involvement of NMDA and non-NMDA glutamate receptors. J. Neurochem 71, 1544–1553 (1998).
Almaas, R., Saugstad, O. D., Pleasure, D. & Rootwelt, T. Neuronal formation of free radicals plays a minor role in hypoxic cell death in human NT2-N neurons. Pediatr. Res. 51, 136–143 (2002).
Ferri, D. et al. Ontogeny, distribution, and possible functional implications of an unusual aquaporin, AQP8, in mouse liver. Hepatology 38, 947–957 (2003).
Suh, H. N. et al. High glucose induced translocation of Aquaporin8 to chicken hepatocyte plasma membrane: involvement of cAMP, PI3K/Akt, PKC, MAPKs, and microtubule. J. Cell. Biochem. 103, 1089–1100 (2008).
Carreras, F. I. et al. Rat hepatocyte aquaporin-8 water channels are down-regulated in extrahepatic cholestasis. Hepatology 37, 1026–1033 (2003).
Wang, H., Chen, J., Hollister, K., Sowers, L. C. & Forman, B. M. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol. Cell. 3, 543–553 (1999).
Legendre, C. et al. Drug-metabolising enzymes are down-regulated by hypoxia in differentiated human hepatoma HepaRG cells: HIF-1alpha involvement in CYP3A4 repression. Eur. J. Cancer 45, 2882–2892 (2009).
Vajro, P. et al. Cholestasis in newborn infants with perinatal asphyxia. Acta Paediatr. 86, 895–898 (1997).
Beath, S. V. Hepatic function and physiology in the newborn. Semin. Neonatol. 8, 337–346 (2003).
Herzog, D., Chessex, P., Martin, S. & Alvarez, F. Transient cholestasis in newborn infants with perinatal asphyxia. Can. J. Gastroenterol. 17, 179–182 (2003).
Jacquemin, E., Saliba, E., Blond, M. H., Chantepie, A. & Laugier, J. Liver dysfunction and acute cardiocirculatory failure in children. Eur. J. Pediatr. 151, 731–734 (1992).
Kamiike, W. et al. Correlation between cellular ATP level and bile excretion in the rat liver. Transplant 39, 50–55 (1985).
Nakatani, T., Sakamoto, Y., Ando, H. & Kobayashi, K. Bile and bilirubin excretion in relation to hepatic energy status during hemorrhagic shock and hypoxemia in rabbits. J. Trauma 39, 665–670 (1995).
Jacquemin, E., Lykavieris, P., Chaoui, N., Hadchouel, M. & Bernard, O. J. Transient neonatal cholestasis: origin and outcome. Pediatr 133, 563–567 (1998).
Hermeziu, B. et al. Heterozygous bile salt export pump deficiency: a possible genetic predisposition to transient neonatal cholestasis. J. Pediatr. Gastroenterol. Nutr. 42, 114–116 (2006).
Jacquemin, E. et al. Heterozygous FIC1 deficiency: a new genetic predisposition to transient neonatal cholestasis. J. Pediatr. Gastroenterol. Nutr. 50, 447–449 (2010).
Marrone, J. et al. Adenoviral transfer of human aquaporin-1 gene to rat liver improves bile flow in estrogen-induced cholestasis. Gene Ther. 21, 1058–1064 (2014).
Marrone, J., Danielli, M., Gaspari, C. I. & Marinelli, R. A. Adenovirus-mediated human aquaporin-1 expression in hepatocytes improves lipopolysaccharide-induced cholestasis. IUBMB Life. 69, 978–984 (2017).
**ong, X. et al. Obeticholic acid protects mice against lipopolysaccharide-induced liver injury and inflammation. Biomed. Pharmacother. 96, 1292–1298 (2017).
El Kasmi, K. C. et al. Pharmacologic activation of hepatic farnesoid X receptor prevents parenteral nutrition-associated cholestasis in mice. Hepatology 75, 252–265 (2022).
Funding
N.S.W. has received financial support from University of Oslo. Runar Almaas has received financial support from The Eckbo Foundation.
Author information
Authors and Affiliations
Contributions
N.S.W. participated in conceptualization and design of the study, acquisition and analysis of data, drafted the initial manuscript, and revised the manuscript. M.A-Å., M.M., M.E.C., S.P.H, R.S. participated in acquisition of data, reviewed and revised the manuscript. G.J.C participated in design of the study, analysis of data, reviewed and revised the manuscript. R.A. participated in conceptualization and design of the study, analysis of data, writing and revision of the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Westerberg, N.S., Atneosen-Åsegg, M., Melheim, M. et al. Effect of hypoxia on aquaporins and hepatobiliary transport systems in human hepatic cells. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03368-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03368-0
- Springer Nature America, Inc.