Abstract
Background
Term and late preterm infants are not routinely referred to high-risk infant follow-up programs at neonatal intensive care unit (NICU) discharge. We aimed to identify NICU factors associated with abnormal developmental screening and develop a risk-stratification model using machine learning for high-risk infant follow-up enrollment.
Methods
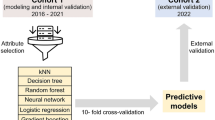
We performed a retrospective cohort study identifying abnormal developmental screening prior to 6 years of age in infants born ≥34 weeks gestation admitted to a level IV NICU. Five machine learning models using NICU predictors were developed by classification and regression tree (CART), random forest, gradient boosting TreeNet, multivariate adaptive regression splines (MARS), and regularized logistic regression analysis. Performance metrics included sensitivity, specificity, accuracy, precision, and area under the receiver operating curve (AUC).
Results
Within this cohort, 87% (1183/1355) received developmental screening, and 47% had abnormal results. Common NICU predictors across all models were oral (PO) feeding, follow-up appointments, and medications prescribed at NICU discharge. Each model resulted in an AUC > 0.7, specificity >70%, and sensitivity >60%.
Conclusion
Stratification of developmental risk in term and late preterm infants is possible utilizing machine learning. Applying machine learning algorithms allows for targeted expansion of high-risk infant follow-up criteria.
Impact
-
This study addresses the gap in knowledge of developmental outcomes of infants ≥34 weeks gestation requiring neonatal intensive care.
-
Machine learning methodology can be used to stratify early childhood developmental risk for these term and late preterm infants.
-
Applying the classification and regression tree (CART) algorithm described in the study allows for targeted expansion of high-risk infant follow-up enrollment to include those term and late preterm infants who may benefit most.



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Manuck, T. A. et al. Preterm neonatal morbidity and mortality by gestational age: a contemporary cohort. Am. J. Obstet. Gynecol. 215, 103.e101–103.e114 (2016).
Harrison, W. & Goodman, D. Epidemiologic trends in neonatal intensive care, 2007-2012. JAMA Pediatr. 169, 855–862 (2015).
Braun, D. et al. Trends in neonatal intensive care unit utilization in a large integrated health care system. JAMA Netw. Open 3, e205239 (2020).
Jarjour, I. T. Neurodevelopmental outcome after extreme prematurity: a review of the literature. Pediatr. Neurol. 52, 143–152 (2015).
Raju, T. N., Higgins, R. D., Stark, A. R. & Leveno, K. J. Optimizing care and outcome for late-preterm (near-Term) infants: a summary of the workshop sponsored by the National Institute of Child Health and Human Development. Pediatrics 118, 1207–1214 (2006).
Gurka, M. J., LoCasale-Crouch, J. & Blackman, J. A. Long-term cognition, achievement, socioemotional, and behavioral development of healthy late-preterm infants. Arch. Pediatr. Adolesc. Med. 164, 525–532 (2010).
Subedi, D., DeBoer, M. D. & Scharf, R. J. Developmental trajectories in children with prolonged NICU stays. Arch. Dis. Child 102, 29–34 (2017).
van Wassenaer‐Leemhuis, A. G. et al. Rethinking preventive post‐discharge intervention programmes for very preterm infants and their parents. Dev. Med. Child Neurol. 58, 67–73 (2016).
Santos, J., Pearce, S. E. & Stroustrup, A. Impact of hospital-based environmental exposures on neurodevelopmental outcomes of preterm infants. Curr. Opin. Pediatr. 27, 254–260 (2015).
Synnes, A. & Hicks, M. Neurodevelopmental outcomes of preterm children at school age and beyond. Clin. Perinatol. 45, 393–408 (2018).
Williams, C. N., Kirby, A. & Piantino, J. If you build it, they will come: initial experience with a multi-disciplinary pediatric neurocritical care follow-up clinic. Children (Basel) 4, 83 (2017).
Vohr, B. et al. Follow-up care of high-risk infants. Pediatrics 114, 1377–1397 (2004).
McAdams, R. M. et al. Predicting clinical outcomes using artificial intelligence and machine learning in neonatal intensive care units: a systematic review. J. Perinatol. 42, 1561–1575 (2022).
Mangold, C. et al. Machine learning models for predicting neonatal mortality: a systematic review. Neonatology 118, 394–405 (2021).
Hathaway, Q. A. et al. Machine-learning to stratify diabetic patients using novel cardiac biomarkers and integrative genomics. Cardiovasc. Diabetol. 18, 78 (2019).
Cheraghlou, S., Sadda, P., Agogo, G. O. & Girardi, M. A machine-learning modified cart algorithm informs Merkel cell carcinoma prognosis. Australas. J. Dermatol 62, 323–330 (2021).
Sheikhtaheri, A., Zarkesh, M. R., Moradi, R. & Kermani, F. Prediction of neonatal deaths in NICUs: development and validation of machine learning models. BMC Med Inf. Decis. Mak. 21, 131 (2021).
Guedalia, J. et al. Primary risk stratification for neonatal jaundice among term neonates using machine learning algorithm. Early Hum. Dev. 165, 105538 (2022).
Van Laere, D. et al. Machine learning to support hemodynamic intervention in the neonatal intensive care unit. Clin. Perinatol. 47, 435–448 (2020).
Boyle, C. A. et al. Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics 127, 1034–1042 (2011).
Holmes, J. F. et al. Validation of a prediction rule for the identification of children with intra-abdominal injuries after Blunt Torso Trauma. Ann. Emerg. Med 54, 528–533 (2009).
Eisenbrown, K., Nimmer, M., Ellison, A. M., Simpson, P. & Brousseau, D. C. Which febrile children with sickle cell disease need a chest x-ray? Acad. Emerg. Med 23, 1248–1256 (2016).
Rigatti, S. J. Random forest. J. Insur Med. 47, 31–39 (2017).
Zhang, Z., Zhao, Y., Canes, A., Steinberg, D. & Lyashevska, O. Predictive analytics with gradient boosting in clinical medicine. Ann. Transl. Med. 7, 152 (2019).
Lu, R. et al. The application of multivariate adaptive regression splines in exploring the influencing factors and predicting the prevalence of Hba1c improvement. Ann. Palliat. Med. 10, 1296–1303 (2021).
Brathwaite, R. et al. Predicting the individualized risk of poor adherence to art medication among adolescents living with Hiv in Uganda: The Suubi+Adherence study. J. Int. AIDS Soc. 24, e25756 (2021).
Glinianaia, S. V. et al. Long-term survival of children born with congenital anomalies: a systematic review and meta-analysis of population-based studies. PLoS Med. 17, e1003356 (2020).
Awad, A., Bader-El-Den, M., McNicholas, J. & Briggs, J. Early hospital mortality prediction of intensive care unit patients using an ensemble learning approach. Int. J. Med Inf. 108, 185–195 (2017).
Ye, C. et al. A real-time early warning system for monitoring inpatient mortality risk: prospective study using electronic medical record data. J. Med Internet Res 21, e13719 (2019).
Ambalavanan, N. & Carlo, W. A. Comparison of the prediction of extremely low birth weight neonatal mortality by regression analysis and by neural networks. Early Hum. Dev. 65, 123–137 (2001).
Ambalavanan, N. et al. Prediction of death for extremely low birth weight neonates. Pediatrics 116, 1367–1373 (2005).
Warren, M. G. et al. Gastrostomy tube feeding in extremely low birthweight infants: frequency, associated comorbidities, and long-term outcomes. J. Pediatr. 214, 41–46.e45 (2019).
Lagatta, J. M. et al. Actual and potential impact of a home nasogastric tube feeding program for infants whose neonatal intensive care unit discharge is affected by delayed oral feedings. J. Pediatr. 234, 38–45.e32 (2021).
Patra, K. & Greene, M. M. Health care utilization after NICU discharge and neurodevelopmental outcome in the first 2 years of life in preterm infants. Am. J. Perinatol. 35, 441–447 (2018).
Gaglioti, P. et al. Fetal cerebral ventriculomegaly: outcome in 176 cases. Ultrasound Obstet. Gynecol. 25, 372–377 (2005).
Brosig, C. L. et al. Preschool neurodevelopmental outcomes in children with congenital heart disease. J. Pediatr. 183, 80–86.e81 (2017).
Patra, K. & Greene, M. M. Impact of feeding difficulties in the NICU on neurodevelopmental outcomes at 8 and 20 months corrected age in extremely low gestational age infants. J. Perinatol. 39, 1241–1248 (2019).
Giannì, M. L. et al. Effect of co-morbidities on the development of oral feeding ability in pre-term infants: a retrospective study. Sci. Rep. 5, 16603 (2015).
Wolthuis-Stigter, M. I. et al. The association between sucking behavior in preterm infants and neurodevelopmental outcomes at 2 years of age. J. Pediatr. 166, 26–30.e21 (2015).
Funding
This project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Numbers UL1TR001436 and TL1TR001437. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Contributions
All authors (K.C., J.Z., E.C., S.H., J.K., K.Y., and S.C.) made substantial contributions to conception, design, and data acquisition, analysis, and interpretation for this manuscript. Authors K.C., E.C., and S.C. drafted the article with authors J.Z., S.H., J.K., and K.Y. providing revisions critically important for the manuscript’s intellectual content. All authors provided final approval of this version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Carlton, K., Zhang, J., Cabacungan, E. et al. Machine learning risk stratification for high-risk infant follow-up of term and late preterm infants. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03338-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03338-6
- Springer Nature America, Inc.