Abstract
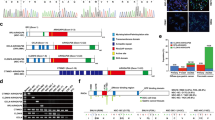
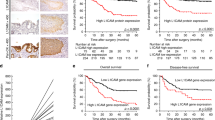
Gastric cancer is the third most common cause of cancer-related death worldwide. Diffuse-type gastric cancer (DGC) is a particularly aggressive subtype that is both difficult to detect and treat. DGC is distinguished by weak cell–cell cohesion, most often due to loss of the cell adhesion protein E-cadherin, a common occurrence in highly invasive, metastatic cancer cells. In this study, we demonstrate that loss-of-function mutation of E-cadherin in DGC cells results in their increased sensitivity to the non-apoptotic, iron-dependent form of cell death, ferroptosis. Homophilic contacts between E-cadherin molecules on adjacent cells suppress ferroptosis through activation of the Hippo pathway. Furthermore, single nucleotide mutations observed in DGC patients that ablate the homophilic binding capacity of E-cadherin reverse the ability of E-cadherin to suppress ferroptosis in both cell culture and xenograft models. Importantly, although E-cadherin loss in cancer cells is considered an essential event for epithelial-mesenchymal transition and subsequent metastasis, we found that circulating DGC cells lacking E-cadherin expression possess lower metastatic ability, due to their increased susceptibility to ferroptosis. Together, this study suggests that E-cadherin is a biomarker predicting the sensitivity to ferroptosis of DGC cells, both in primary tumor tissue and in circulation, thus guiding the usage of future ferroptosis-inducing therapeutics for the treatment of DGC.






Similar content being viewed by others
Data availability
All raw data values and Western blots are available as Supplementary Data. All other relevant data are available upon request from the authors.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–62.
Ansari S, Gantuya B, Tuan VP, Yamaoka Y. Diffuse gastric cancer: a summary of analogous contributing factors for its molecular pathogenicity. Int J Mol Sci. 2018;19:2424.
Liu X, Chu K-M. E-cadherin and gastric cancer: cause, consequence, and applications. Biomed Res Int. 2014;2014:637308.
Kaurah P, MacMillan A, Boyd N, Senz J, De Luca A, Chun N, et al. Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA. 2007;297:2360–72.
Till JE, Yoon C, Kim B-J, Roby K, Addai P, Jonokuchi E, et al. Oncogenic KRAS and p53 loss drive gastric tumorigenesis in mice that can be attenuated by E-cadherin expression. Cancer Res. 2017;77:5349–59.
Lewis FR, Mellinger JD, Hayashi A, Lorelli D, Monaghan KG, Carneiro F, et al. Prophylactic total gastrectomy for familial gastric cancer. Surgery. 2001;130:612–7. discussion 617–9.
Shenoy S. CDH1 (E-cadherin) mutation and gastric cancer: genetics, molecular mechanisms and guidelines for management. Cancer Manag Res. 2019;11:10477–86.
Pandalai PK, Lauwers GY, Chung DC, Patel D, Yoon SS. Prophylactic total gastrectomy for individuals with germline CDH1 mutation. Surgery. 2011;149:347–55.
Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239–48.
van Roy F, Berx G. The cell-cell adhesion molecule E-cadherin. Cell Mol Life Sci. 2008;65:3756–88.
Perez-Moreno M, Fuchs E. Catenins: kee** cells from getting their signals crossed. Dev Cell. 2006;11:601–12.
Koch AW, Manzur KL, Shan W. Structure-based models of cadherin-mediated cell adhesion: the evolution continues. Cell Mol Life Sci. 2004;61:1884–95.
Shapiro L, Fannon AM, Kwong PD, Thompson A, Lehmann MS, Grübel G, et al. Structural basis of cell-cell adhesion by cadherins. Nature. 1995;374:327–37.
Parisini E, Higgins JM, Liu JH, Brenner MB, Wang JH. The crystal structure of human E-cadherin domains 1 and 2, and comparison with other cadherins in the context of adhesion mechanism. J Mol Biol. 2007;373:401–11.
Boggon TJ, Murray J, Chappuis-Flament S, Wong E, Gumbiner BM, Shapiro L. C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science. 2002;296:1308–13.
Brieher WM, Yap AS, Gumbiner BM. Lateral dimerization is required for the homophilic binding activity of C-cadherin. J Cell Biol. 1996;135:487–96.
Wu J, Minikes AM, Gao M, Bian H, Li Y, Stockwell BR, et al. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature. 2019;572:402–6.
Kim NG, Koh E, Chen X, Gumbiner BM. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci USA. 2011;108:11930–5.
Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30:1–17.
Kim TY, Jong H-S, Jung Y, Kim T-Y, Kang GH, Bang Y-J. DNA hypermethylation in gastric cancer. Aliment Pharmacol Ther. 2004;20:131–42.
Handschuh G, Candidus S, Luber B, Reich U, Schott C, Oswald S, et al. Tumour-associated E-cadherin mutations alter cellular morphology, decrease cellular adhesion and increase cellular motility. Oncogene. 1999;18:4301–12.
Figueiredo J, Ferreira RM, Xu H, Gonçalves M, Barros-Carvalho A, Cravo J, et al. Integrin β1 orchestrates the abnormal cell-matrix attachment and invasive behaviour of E-cadherin dysfunctional cells. Gastric Cancer. 2022;25:124–37.
Yang WH, Ding CC, Sun T, Rupprecht G, Lin CC, Hsu D, et al. The Hippo pathway effector TAZ regulates ferroptosis in renal cell carcinoma. Cell Rep. 2019;28:2501.e2504.
Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell. 2015;59:298–308.
Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13:81–90.
Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, et al. ACSL4 dictates ferroptosis sensitivity by sha** cellular lipid composition. Nat Chem Biol. 2017;13:91–8.
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72.
Qin Y, Pei Z, Feng Z, Lin P, Wang S, Li Y, et al. Oncogenic activation of YAP signaling sensitizes ferroptosis of hepatocellular carcinoma via ALOXE3-mediated lipid peroxidation accumulation. Front Cell Dev Biol. 2021;9:751593.
Padmanaban V, Krol I, Suhail Y, Szczerba BM, Aceto N, Bader JS, et al. E-cadherin is required for metastasis in multiple models of breast cancer. Nature. 2019;573:439–44.
Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27–40.
Yuan H, Li X, Zhang X, Kang R, Tang D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun. 2016;478:1338–43.
Feng H, Schorpp K, ** J, Yozwiak CE, Hoffstrom BG, Decker AM, et al. Transferrin receptor is a specific ferroptosis marker. Cell Rep. 2020;30:3411–23.e3417.
Hangauer MJ, Viswanathan VS, Ryan MJ, Bole D, Eaton JK, Matov A, et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature. 2017;551:247–50.
Viswanathan VS, Ryan MJ, Dhruv HD, Gill S, Eichhoff OM, Seashore-Ludlow B, et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature. 2017;547:453–7.
Yamaguchi H, Taouk GM. A potential role of YAP/TAZ in the interplay between metastasis and metabolic alterations. Front Oncol (Rev). 2020;10:928.
Heerboth S, Housman G, Leary M, Longacre M, Byler S, Lapinska K, et al. EMT and tumor metastasis. Clin Transl Med. 2015;4:6.
Ubellacker JM, Tasdogan A, Ramesh V, Shen B, Mitchell EC, Martin-Sandoval MS, et al. Lymph protects metastasizing melanoma cells from ferroptosis. Nature. 2020;585:113–8.
Padmanaban V, Krol I, Suhail Y, Szczerba BM, Aceto N, Bader JS, et al. E-cadherin is required for metastasis in multiple models of breast cancer. Nature. 2019;573:439–44.
Forcina GC, Conlon M, Wells A, Cao JY, Dixon SJ. Systematic quantification of population cell death kinetics in mammalian cells. Cell Syst. 2017;4:600–10.e606.
Acknowledgements
The authors thank members of the Jiang lab for critical reading and suggestions. This work is supported by NIH F31CA247112 (to AMM), NIH R01CA204232 and NIH R01CA258622 (to XJ), and NCI cancer center core grant P30 CA008748 to MSKCC.
Author information
Authors and Affiliations
Contributions
AMM, YS, and XJ conceived and designed the study. AMM performed most of the experiments, with contributions from YS and CY. CY and SSY contributed materials and advice. AMM, YS, and XJ performed data analysis and wrote the manuscript. All authors contributed to the writing and editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
XJ and AMM are inventors of patents relevant to ferroptosis and autophagy. XJ is a consultant and equity holder of Exarta Therapeutics and Lime Therapeutics. The rest of the authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Minikes, A.M., Song, Y., Feng, Y. et al. E-cadherin is a biomarker for ferroptosis sensitivity in diffuse gastric cancer. Oncogene 42, 848–857 (2023). https://doi.org/10.1038/s41388-023-02599-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-023-02599-5
- Springer Nature Limited
This article is cited by
-
The multifaceted perspectives on the regulation of lncRNAs in hepatocellular carcinoma ferroptosis: from bench-to-bedside
Clinical and Experimental Medicine (2024)