Abstract
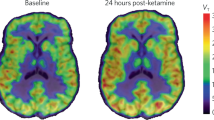
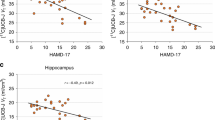
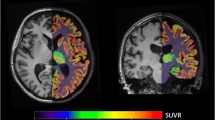
The discovery of ketamine as a rapid and robust antidepressant marks the beginning of a new era in the treatment of psychiatric disorders. Ketamine is thought to produce rapid and sustained antidepressant effects through restoration of lost synaptic connections. We investigated this hypothesis in humans for the first time using positron emission tomography (PET) and [11C]UCB-J—a radioligand that binds to the synaptic vesicle protein 2A (SV2A) and provides an index of axon terminal density. Overall, we did not find evidence of a measurable effect on SV2A density 24 h after a single administration of ketamine in non-human primates, healthy controls (HCs), or individuals with major depressive disorder (MDD) and/or posttraumatic stress disorder (PTSD), despite a robust reduction in symptoms. A post-hoc, exploratory analysis suggests that patients with lower SV2A density at baseline may exhibit increased SV2A density 24 h after ketamine. This increase in SV2A was associated with a reduction in depression severity, as well as an increase in dissociative symptoms. These initial findings suggest that a restoration of synaptic connections in patients with lower SV2A at baseline may underlie ketamine’s therapeutic effects, however, this needs replication in a larger sample. Further work is needed to build on these initial findings and further establish the nuanced pre- and post-synaptic mechanisms underpinning ketamine’s therapeutic effects.




Similar content being viewed by others
References
Kraus C, Wasserman D, Henter ID, Acevedo-Diaz E, Kadriu B, Zarate CA Jr. The influence of ketamine on drug discovery in depression. Drug Discov Today. 2019;24:2033–43. https://doi.org/10.1016/j.drudis.2019.07.007
World Health Organization, Depression and other common mental disorders: global health estimates. (World Health Organization, 2017).
Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, Rush AJ. What did STAR* D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. 2009;60:1439–45.
Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77.
Krystal JH, Abdallah CG, Sanacora G, Charney DS, Duman RS. Ketamine: a paradigm shift for depression research and treatment. Neuron. 2019;101:774–8.
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–4. https://doi.org/10.1016/S0006-3223(99)00230-9
Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–64.
Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB, et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172:950–66.
Kaufman MB. Pharmaceutical approval update. Pharm Ther. 2019;44:42–4.
Feder A, Parides MK, Murrough JW, Perez AM, Morgan JE, Saxena S, et al. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2014;71:681–8.
Albott CS, Lim KO, Forbes MK, Erbes C, Tye SJ, Grabowski JG, et al. Efficacy, safety, and durability of repeated ketamine infusions for comorbid posttraumatic stress disorder and treatment-resistant depression. J Clin Psychiatry. 2018;79:17462.
Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793–802.
Zarate CA Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71:939–46.
Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22:238–49.
Duman RS. Neurobiology of stress, depression, and rapid-acting antidepressants: remodeling synaptic connections. Depress Anxiety. 2014;31:291–6.
Krystal JH, Abdallah CG, Averill LA, Kelmendi B, Harpaz-Rotem I, Sanacora G, et al. Synaptic loss and the pathophysiology of PTSD: implications for ketamine as a prototype novel therapeutic. Curr Psychiatry Rep. 2017;19:74.
Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacol: Off Publ Am Coll Neuropsychopharmacol. 2010;35:192–216.
Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72:603–11.
Kühn S, Gallinat J. Gray matter correlates of posttraumatic stress disorder: a quantitative meta-analysis. Biol Psychiatry. 2013;73:70–74.
Akiki TJ, Averill CL, Abdallah CG. A network-based neurobiological model of PTSD: evidence from structural and functional neuroimaging studies. Curr Psychiatry Rep. 2017;19:81.
Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012;18:1413–7.
Holmes SE, Scheinost D, Finnema SJ, Naganawa M, Davis MT, DellaGioia N, et al. Lower synaptic density is associated with depression severity and network alterations. Nat Commun. 2019;10:1–10.
Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–61.
Shansky RM, Morrison JH. Stress-induced dendritic remodeling in the medial prefrontal cortex: effects of circuit, hormones and rest. Brain Res. 2009;1293:108–13.
Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–20.
Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6.
Banasr M, Valentine GW, Li XY, Gourley SL, Taylor JR, Duman RS. Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol Psychiatry. 2007;62:496–504.
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–64.
Duman RS, Li N, Liu R-J, Duric V, Aghajanian G. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 2012;62:35–41.
Abdallah CG, Sanacora G, Duman RS, Krystal JH. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med. 2015;66:509–23.
Kavalali ET, Monteggia LM. Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am J Psychiatry. 2012;169:1150–6.
Ali F, Gerhard DM, Sweasy K, Pothula S, Pittenger C, Duman RS, et al. Ketamine disinhibits dendrites and enhances calcium signals in prefrontal dendritic spines. Nat Commun. 2020;11:72 https://doi.org/10.1038/s41467-019-13809-8
Widman AJ, McMahon LL. Disinhibition of CA1 pyramidal cells by low-dose ketamine and other antagonists with rapid antidepressant efficacy. Proc Natl Acad Sci. 2018;115:E3007–E3016.
Abdallah CG, De Feyter HM, Averill LA, Jiang L, Averill CL, Chowdhury G, et al. The effects of ketamine on prefrontal glutamate neurotransmission in healthy and depressed subjects. Neuropsychopharmacol: Off Publ Am Coll Neuropsychopharmacol. 2018;43:2154–60.
Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res. 2011;224:107–11.
Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95.
Liu R-J, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry. 2012;71:996–1005.
Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–27.
Ota KT, Liu RJ, Voleti B, Maldonado-Aviles JG, Duric V, Iwata M, et al. REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat Med. 2014;20:531–5.
Moda-Sava, RN, Murdock MH, Parekh PK, Fetcho RN, Huang BS, Huynh TN, et al. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science. 2019;364:8078.
Finnema SJ, Nabulsi NB, Eid T, Detyniecki K, Lin SF, Chen MK, et al. Imaging synaptic density in the living human brain. Sci Transl Med. 2016;8:348ra396–348ra396.
Bajjalieh SM, Frantz G, Weimann JM, McConnell SK, Scheller R. Differential expression of synaptic vesicle protein 2 (SV2) isoforms. J Neurosci. 1994;14:5223–35.
Kishimoto T, Chawla JM, Hagi K, Zarate CA, Kane JM, Bauer M, et al. Single-dose infusion ketamine and non-ketamine N-methyl-d-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychological Med. 2016;46:1459–72.
Fukumoto K, Fogaça MV, Liu RJ, Duman C, Kato T, Li XY, et al. Activity-dependent brain-derived neurotrophic factor signaling is required for the antidepressant actions of (2R, 6R)-hydroxynorketamine. Proc Natl Acad Sci. 2019;116:297–302.
Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, et al. Measurement of dissociative states with the clinician‐administered dissociative states scale (CADSS). J Trauma Stress: Off Publ Int Soc Trauma Stress Stud. 1998;11:125–36.
Hamilton M. A rating scale for depression. J Neurol, Neurosurg Psychiatry. 1960;23:56–62.
Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9.
Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100.
Finnema SJ, Nabulsi NB, Mercier J, Lin S-f, Chen M-K, Matuskey D, et al. Kinetic evaluation and test-retest reproducibility of [11C] UCB-J, a novel radioligand for positron emission tomography imaging of synaptic vesicle glycoprotein 2A in humans. J Cereb Blood Flow Metab. 2018;38:2041–52.
Nabulsi NB, Mercier J, Holden D, Carré S, Najafzadeh S, Vandergeten MC, et al. Synthesis and preclinical evaluation of 11C-UCB-J as a PET tracer for imaging the synaptic vesicle glycoprotein 2A in the brain. J Nucl Med. 2016;57:777–84.
Rohlfing T, Kroenke CD, Sullivan EV, Dubach MF, Bowden DM, Grant KA, et al. The INIA19 template and neuromaps atlas for primate brain image parcellation and spatial normalization. Front Neuroinformatics. 2012;6:27 https://doi.org/10.3389/fninf.2012.00027
Sandiego CM, Weinzimmer D, Carson RE. Optimization of PET–MR registrations for nonhuman primates using mutual information measures: a Multi-Transform Method (MTM). Neuroimage. 2013;64:571–81.
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214.
Esterlis I, DellaGioia N, Pietrzak RH, Matuskey D, Nabulsi N, Abdallah CG, et al. Ketamine-induced reduction in mGluR5 availability is associated with an antidepressant response: an [11 C] ABP688 and PET imaging study in depression. Mol Psychiatry. 2018;23:824–32.
Holmes SE, Gallezot JD, Davis MT, DellaGioia N, Matuskey D., Nabulsi N, et al.. Measuring the effects of ketamine on mGluR5 using [18F] FPEB and PET. 2019;40:2254–64.
Luckenbaugh DA, Niciu MJ, Ionescu DF, Nolan NM, Richards EM, Brutsche NE, et al. Do the dissociative side effects of ketamine mediate its antidepressant effects? J Affect Disord. 2014;159:56–61.
Yaden, DB & Griffiths, RR. The subjective effects of psychedelics are necessary for their enduring therapeutic effects. ACS Pharmacol Transl Sci., https://doi.org/10.1021/acsptsci.0c00194 (2020).
Fava M, Freeman MP, Flynn M, Judge H, Hoeppner BB, Cusin C, et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry. 2020;25:1592–603.
Ingram R, Kang H, Lightman S, Jane DE, Bortolotto ZA, Collingridge GL, et al. Some distorted thoughts about ketamine as a psychedelic and a novel hypothesis based on NMDA receptor-mediated synaptic plasticity. Neuropharmacology. 2018;142:30–40. https://doi.org/10.1016/j.neuropharm.2018.06.008
Müller HK, Wegener G, Liebenberg N, Zarate CA Jr, Popoli M, Elfving B. Ketamine regulates the presynaptic release machinery in the hippocampus. J Psychiatr Res. 2013;47:892–9.
Bhatt DH, Zhang S, Gan W-B. Dendritic spine dynamics. Annu Rev Physiol. 2009;71:261–82.
Chowdhury GM, Zhang J, Thomas M, Banasr M, Ma X, Pittman B, et al. Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects. Mol Psychiatry. 2017;22:120–6.
Lazarevic V, Yang Y, Flais I & Svenningsson P. Ketamine decreases neuronally released glutamate via retrograde stimulation of presynaptic adenosine A1 receptors. Molecular Psychiatry. 2021;21:1–11.
Vitureira N, Letellier M, Goda Y. Homeostatic synaptic plasticity: from single synapses to neural circuits. Curr Opin Neurobiol. 2012;22:516–21. https://doi.org/10.1016/j.conb.2011.09.006
Kato T, Pothula S, Liu RJ, Duman CH, Terwilliger R, Vlasuk GP, et al. Sestrin modulator NV-5138 produces rapid antidepressant effects via direct mTORC1 activation. J Clin Investig. 2019;129:2542–54. https://doi.org/10.1172/JCI126859
Li C-T, Chen MH, Lin WC, Hong CJ, Yang BH, Liu RS, et al. The effects of low-dose ketamine on the prefrontal cortex and amygdala in treatment-resistant depression: a randomized controlled study. Hum Brain Mapp. 2016;37:1080–90. https://doi.org/10.1002/hbm.23085
Nugent AC, Ballard ED, Gould TD, Park LT, Moaddel R, Brutsche NE, et al. Ketamine has distinct electrophysiological and behavioral effects in depressed and healthy subjects. Mol Psychiatry. 2019;24:1040–52. https://doi.org/10.1038/s41380-018-0028-2
Acknowledgements
The authors dedicate this work to the late Ronald S. Duman—a colleague, mentor, and friend whose pioneering research has made seminal contributions to the neuroscience of depression and PTSD, and to understanding the mechanisms underlying ketamine’s therapeutic effects. We thank the staff at Yale PET Center and the West Haven National Center for PTSD, and all the individuals who took part in this study. Funding support was provided by the Veterans Affairs National Center for PTSD (RSD, JHK, and IE), the National Center for Advancing Translational Science (SEH: UL1TR001863), the Nancy Taylor Foundation (IE), and the NARSAD Young Investigator Award (SJF).
Author information
Authors and Affiliations
Contributions
IE, RSD, JHK, and GS conceived and designed the experiments. SEH analyzed the PET data with input from REC, SJF, and MN. The manuscript was written by SEH with feedback from co-authors. Non-human primate imaging was conducted by DH and KF. Radiochemistry was performed by JR, PE, YY, and NN. Recruitment was overseen by ND and MD. GS assisted with the referral of clinical participants and interpretation of findings. DM and GAA provided medical oversight. All authors helped shape the research, analysis, and manuscript.
Corresponding authors
Ethics declarations
Competing interests
Dr. Krystal acknowledges the following relevant financial interests. He is a co-sponsor of a patent for the intranasal administration of ketamine for the treatment of depression that was licensed by Janssen Pharmaceuticals, the maker of s-ketamine. He has a patent related to the use of riluzole to treat anxiety disorders that were licensed by Biohaven Pharmaceuticals Medical Sciences. He has stock or stock options in Biohaven Pharmaceuticals Medical Sciences, Sage Pharmaceuticals, Spring Care Inc., EpiVario Inc., Neumora Therapeutics Inc., Terran Biosciences Inc., and Tempero Bio, Inc. He consults broadly to the pharmaceutical industry, but his annual income over the past year did not exceed $5000 for any organization. He receives over $5000 in income from the Society of Biological Psychiatry for editing the journal Biological Psychiatry. Dr. Gerard Sanacora has served as consultant to Allergan, Alkermes, AstraZeneca, Avanier Pharmaceuticals, Axsome Therapeutics, Biogen, Biohaven Pharmaceuticals, Boehringer Ingelheim International GmbH, Bristol-Myers Squibb, Cowen, EMA Wellness, Engrail Therapeutics, Clexio, Denovo Biopharma, Gilgamesh, Hoffman La-Roche, Intra-Cellular Therapies, Janssen, Levo, Lundbeck, Merck, Navitor Pharmaceuticals, Neurocrine, Novartis, Noven Pharmaceuticals, Otsuka, Perception Neuroscience, Praxis Therapeutics, Sage Pharmaceuticals, Servier Pharmaceuticals, Seelos Pharmaceuticals, Taisho Pharmaceuticals, Teva, Valeant, Vistagen Therapeutics, and XW Labs; and received research contracts from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Johnson & Johnson, Merck, Naurex, and Usona over the past 36 months. Dr. Sanacora holds equity in BioHaven Pharmaceuticals and is a co-inventor on a US patent (#8778979) held by Yale University and a co-inventor on US Provisional Patent Application No. 047162-7177P1 (00754) filed on August 20, 2018, by Yale University Office of Cooperative Research. Yale University has a financial relationship with Janssen Pharmaceuticals and may in the future receive financial benefits from this relationship. The University has put multiple measures in place to mitigate this institutional conflict of interest. Questions about the details of these measures should be directed to Yale University’s Conflict of Interest office. Dr. Sjoerd Finnema is an employee and shareholder of Abbvie. None of the authors declare any conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Holmes, S.E., Finnema, S.J., Naganawa, M. et al. Imaging the effect of ketamine on synaptic density (SV2A) in the living brain. Mol Psychiatry 27, 2273–2281 (2022). https://doi.org/10.1038/s41380-022-01465-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01465-2
- Springer Nature Limited
This article is cited by
-
A systematic review and meta-analysis of neuroimaging studies examining synaptic density in individuals with psychotic spectrum disorders
BMC Psychiatry (2024)
-
Ketamine and rapid antidepressant action: new treatments and novel synaptic signaling mechanisms
Neuropsychopharmacology (2024)
-
Substance use and spine density: a systematic review and meta-analysis of preclinical studies
Molecular Psychiatry (2024)
-
Ketamine in neuropsychiatric disorders: an update
Neuropsychopharmacology (2024)
-
Effects of escitalopram on synaptic density in the healthy human brain: a randomized controlled trial
Molecular Psychiatry (2023)