Abstract
Flexible multichannel electrode arrays (fMEAs) with multiple filaments can be flexibly implanted in various patterns. It is necessary to develop a method for implanting the fMEA in different locations and at various depths based on the recording demands. This study proposed a strategy for reducing the microelectrode volume with integrated packaging. An implantation system was developed specifically for semiautomatic distributed implantation. The feasibility and convenience of the fMEA and implantation platform were verified in rodents. The acute and chronic recording results provied the effectiveness of the packaging and implantation methods. These methods could provide a novel strategy for develo** fMEAs with more filaments and recording sites to measure functional interactions across multiple brain regions.
Similar content being viewed by others
Introduction
Microelectrode arrays made of silicon or other stiff materials have to be implanted with the original topology1,2,3,4,5. A flexible multichannel electrode array (fMEA) with the same topology can be placed in the cortex at various positions within a certain range. The distance between the filaments and the depth of each filament can be adjusted based on the requirements6,7. Because of its flexibility, the fMEA can be implanted in various brain regions. We term this process distributed implantation. Direct implantation is infeasible due to the lack of required stiffness. Other materials or methods must be used to assist with fMEA implantation8,9,10,11,12,13. The injection is a promising implantation method. However, current injection technology requires complex preparation and a complicated packaging process after implantation, and the brain is vulnerable to overpressure14,15,16,17,18,19. Reinforced materials are popular for assisting with fMEA implantation. Degradable materials such as polyethylene glycol (PEG) or fibroin can be coated on the outside of the flexible filament to temporarily reinforce the stiffness20,21,22. After implantation, the coating material degrades and is absorbed by body tissue. However, in many cases, the stiffness of the reinforcing material is insufficient, which increases the difficulty of implantation. The reinforcing material needs to degrade and be absorbed, which may take time and result in toxicity23,24. Another popular method involves inserting an fMEA into the cortex with stiff shuttling probes or microneedles7,8,9,10,25,26. The fMEA filament is fixed on a shuttle probe6,26,27. However, there is usually more than one leg or filament on the fMEA7,11. In that case, complicated manual operations are required to individually align and fix the flexible shank on the shuttle probe26. Another option is to simultaneously align several filaments to the same number of shuttle probes and glue them together7. However, as the number of shanks increases, the current operational methods become incapable of implanting them effectively and safely. At present, neither the reinforced material nor the pre-glued shuttle probe satisfies the demands of fMEA distributed implantation. Elon Musk reported an implant known as Robert, which can automatically implant individual fMEA filaments6. However, Robert is too complex and expensive to be widely used.
An fMEA is composed of two parts: the implant part and the connector part. The recording sites are located at the front end of the implant part. The connector part, which is often soldered to a multichannel plug, remains outside and is mounted on the head. A bundle of wire lines connects the fMEA to the socket of a multichannel recording system. A microelectromechanical system (MEMS) process is commonly used to fabricate the fMEA. To minimize the volume of the implant part, the line width and line space of this part can be reduced to one micrometer or the nanometer scale8,9,26,28,29. However, the line spacing and dimension of the connector part must be increased to fit a plug connector11,28. A typical miniature plug connector, such as the Omnetics connector, a common interface for implant electrodes, has a minimum pin spacing of 640 μm. A 32 pin double-row Omnetics connector is 3 mm wide and 10 mm long. As the number of recording sites on the fMEA increases, the connector part can increase to an unreasonably large size28.
A potential solution for improving fMEAs was developed in this paper. To allow for simple and practicable implantation, homemade implanting tools, combined with the guide hole at the front end of the implant filaments, can semiautomatically catch and implant individual fMEA threads. The position and depth of each thread in the fMEA can be controlled with this tool. This reduces the manual operation while improving the implantation accuracy and speed. To reduce the fMEA volume necessary for plug-socket fan-out methods, we propose a direct interconnection method. The specially designed pads at the connector part of the fMEA, combined with the modified gold ball bonding process, connect the fMEA and the amplifier. This method eliminates the need for pairs of plug-socket connectors. The proposed implantation and interconnection methods provide a solution for develo** fMEAs with multiple filaments and recording sites.
Results
Fabrication and packaging of the fMEA
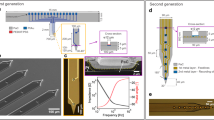
The Polyimide (PI) 2600 series was chosen as the flexible substrate due to its excellent biocompatibility and flexibility. The device has a PI–metal–PI sandwich structure. The fabrication process of the fMEA is shown in Fig. 1a. The fMEA had four filaments and eight recording sites on each filament, as shown in Fig. 1b and Fig. S1. The filament was ~2.5 cm in length. Thus, it was long enough to be implanted in any area of the rat brain using the semiautomatic implantation platform. The width of the filament was only 70 μm to minimize the volume of the implant part. The recording sites were 10 μm long and arranged in a line along the filament. The distance between the recording sites was 100 μm. A loop at the front end of each filament was fabricated as a handle to assist with implantation. The loop was surrounded and marked by a gold ring to facilitate observation. The square bonding pad had a hollow round hole in the center to facilitate small-scale integrated packaging. To ensure that the filament had enough mechanical strength to withstand clam**, dragging, and pulling during the operation, polyimide with a tensile strength of 350 MPa was chosen. Flexible microelectrodes with a thickness of 6 μm were fabricated, and the bending stiffness of the thin-film filament was 1.07 × 10−11 Nm2 (Supplementary Methods). By adjusting the spin-coating parameters, an fMEA with a thickness of 2.4 μm or less can be produced.
a Schematic of the fMEA fabrication steps. (i) Deposition of the aluminum release layer. (ii) Spin-coating and curing of the bottom PI layer. (iii) Photolithographic patterning and growing of the gold layer. (iv) Spin-coating and curing of the top PI layer. (v) Photolithographic patterning and RIE to expose the recording sites and bonding pads. (vi) Photolithographic patterning and RIE to empty the implantation hole at the front end and the hollow welding points on the bonding pads. (vii) Release of the fMEA by aluminum etching. b Comparison photo of a conventional package with an Omnetics connector (left) and the integrated package (right). c Schematics of fMEA encapsulation by gold bonding. d The two-electrode system (left), with an fMEA as the working electrode and platinum(Pt) for the counter electrode and reference electrode. Schematic of electropolymerization of PEDOT (right)
Neural signal acquisition, amplification, digitization, and multiplexing were executed by the amplifier module to ensure signal quality and reduce the number of external leads. We connected the fMEA to the module with a modified gold ball bonding process, as shown in Fig. 1c. The volume of the module was less than half of the volume of a traditional package microelectrode with an Omnetics connector, as shown in Fig. 1b. The proposed fMEA with the amplifier weighs 0.580 g, while the traditional package microelectrode weighs 1.328 g. The small-size integrated packaging solution based on gold ball bonding is easy to operate and can be scaled up to hundreds or more channels.
Impedance test and microtopography observation
Acknowledgements
The project was supported by the National Key Technologies Research and Development Program of China (2017YFA0205903, 2017YFA0701100); the National Natural Science Foundation of China (61634006, 62071447); the Strategic Priority Research Program of Chinese Academy of Sciences Pilot Project (XDB32030102, XDB32040203, XDA16021305); and the Key Research Programs of Frontier Sciences, CAS (QYZDY-SSW-JSC004).
Author information
Authors and Affiliations
Contributions
C.W., W.P., and Y.W. conceived the project. C.W., W.P., Y.W., X.Y., and X.H. designed and performed the experiments. C.W., G.M., and L.Z. performed the data analysis. C.W., L.L., R.C., P.W., and Y.W. contributed to the discussion. C.W. and Z.L. prepared the figures. C.W. and W.P. co-wrote the paper. All authors approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wei, C., Wang, Y., Pei, W. et al. Distributed implantation of a flexible microelectrode array for neural recording. Microsyst Nanoeng 8, 50 (2022). https://doi.org/10.1038/s41378-022-00366-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41378-022-00366-2
- Springer Nature Limited
This article is cited by
-
Implantable Electrochemical Microsensors for In Vivo Monitoring of Animal Physiological Information
Nano-Micro Letters (2024)
-
Flexible multichannel electrodes for acute recording in nonhuman primates
Microsystems & Nanoengineering (2023)
-
Implantable intracortical microelectrodes: reviewing the present with a focus on the future
Microsystems & Nanoengineering (2023)
-
A mosquito mouthpart-like bionic neural probe
Microsystems & Nanoengineering (2023)