Abstract
Objective
To assess the proposed shortened tools based on the Finnegan neonatal abstinence scoring tool (FNAS) for relative clinical utility.
Study design
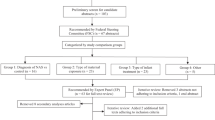
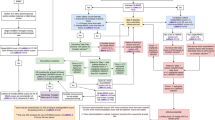
Retrospective study comparing shortened tools with FNAS on need for treatment, medication initiation cutoff score agreement, and length of treatment in 369 infants with prenatal opioid exposure using estimated areas under the receiver operating characteristic curves, Pearson and Spearman correlations, and proportion correctly classified, sensitivity, and specificity.
Results
The tools by Gomez et al. and Chervoneva et al. are most predictive of the FNAS cut-off values to initiate treatment, have cutoff values that best align with the FNAS cutoff values, and strongly correlate with the FNAS (r ≥ 0.88 corresponding to treatment initiation, r ≥ 0.83 during first 10 days of treatment).
Conclusion
The tools of Gomez and Chervoneva demonstrated potential clinical usefulness by strongly associating with the need for treatment and monitoring the course of NAS therapy.
Similar content being viewed by others
References
Bauer A, Li, Y. Neonatal abstinence syndrome and maternal substance abuse in Tennessee: 1999–2011. Nashville, TN: Tennessee Department of Health; 2013.
McQueen K, Murphy-Oikonen J. Neonatal abstinence syndrome. N. Engl J Med. 2016;375:2468–79.
Haight SC, Ko JY, Tong VT, Bohm MK, Callaghan WM. Opioid use disorder documented at delivery hospitalization—United States, 1999–2014. MMWR Morb Mortal Wkly Rep. 2018;67:845–9.
Hirai AH, Ko JY, Owens PL, Stocks C, Patrick SW. Neonatal abstinence syndrome and maternal opioid-related diagnoses in the US, 2010–2017. JAMA 2021;325:146–55.
Patrick SW, Barfield WD, Poindexter BB, Committee on Fetus and Newborn, Committee on Substance Use and Prevention. Neonatal Opioid Withdrawal Syndrome. Pediatrics 2020;146:e2020029074.
Finnegan LP, Kaltenbach K. Neonatal abstinence syndrome. In: Hoekelman RA, Friedman SB, Nelson NM, et al. eds. Primary Pediatric Care. 2nd ed. St. Louis, MO: Mosby; 1992:1367-78.
Finnegan LP, Kron RE, Connaughton JF, Emich JP. Assessment and treatment of abstinence in the infant of the drug drug-dependent mother. Int J Clin Pharm Biopharm. 1975;12:19–32.
Westgate PM, Gomez Pomar E. Judging the neonatal abstinence syndrome assessment tools to guide future tool development: the use of clinimetrics as opposed to psychometrics. Front Pediatrics. 2017;5:204.
Kaltenbach K, Holbrook A, Coyle MG, Heil SH, Salisbury A, Stine S, et al. Predicting treatment for neonatal abstinence syndrome in infants born to women maintained on opioid agonist medication. Addiction 2012;107:45–52.
Maguire D, Cline GJ, Parnell L, Tai CY. Validation of the Finnegan neonatal abstinence syndrome tool-short form. Adv Neonatal Care. 2013;13:430–7.
Gomez Pomar E, Finnegan LP, Devlin L, Bada H, Concina VA, Ibonia KT, et al. Simplification of the Finnegan Neonatal Abstinence Scoring System: retrospective study of two institutions in the USA. BMJ Open. 2017;7:e016176.
Devlin LA, Breeze JL, Terrin N, Gomez Pomar E, Bada H, Finnegan LP, et al. Association of a simplified Finnegan Neonatal abstinence scoring tool with the need for pharmacologic treatment for neonatal abstinence syndrome. JAMA Netw Open 2020;3:e202275.
Chervoneva I, Adeniyi-Jones SC, Blanco F, Kraft WK. Development of an abbreviated symptom score for the neonatal abstinence syndrome. J Perinatol. 2020;40:1031–40. https://doi.org/10.1038/s41372-020-0606-4. Epub 2020 Feb 19
Breiman L Classification and Regression Trees. 1st ed. Boca Raton: Chapman and Hall/CRC; 2017.
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software partners, J Biomed Inform. 2019 May 9.
SAS Institute Inc 2013. SAS/ACCESS® 9.4 Interface to ADABAS: Reference. Cary, NC: SAS Institute Inc.
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2017. https://www.R-project.org/.
Fisher RA. On the “Probable Error” of a coefficient of correlation deduced from a small sample. Metron. 1921;1:3–32.
Hittner JB, May K, Silver NC. A Monte Carlo evaluation of tests for comparing dependent correlations. J Gen Psychol. 2003;130:149–68. https://doi.org/10.1080/00221300309601282. PMID: 12773018.
Diedenhofen B, Musch J. Cocor: a comprehensive solution for the statistical comparison of correlations [published correction appears in PLoS One. 2015;10(6):e0131499]. PLoS One. 2015;10:e0121945 https://doi.org/10.1371/journal.pone.0121945. Published 2015 Apr 2.
Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinforma. 2011;7:77 https://doi.org/10.1186/1471-2105-12-77.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45. PMID: 3203132.
Sun X, Xu W. Fast implementation of DeLongs algorithm for comparing the areas under correlated receiver operating characteristic curves’. IEEE Signal Process Lett. 2014;21:1389–93. https://doi.org/10.1109/LSP.2014.2337313.
Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–13.
McNemar Q. Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika 1947;12:153–7. https://doi.org/10.1007/BF02295996. PMID: 20254758.
Davison AC, Hinkley DV (1997). Bootstrap Methods and Their Applications. Cambridge University Press, Cambridge. ISBN 0-521-57391-2, http://statwww.epfl.ch/davison/BMA/.
Canty A, Ripley BD (2021). boot: Bootstrap R (S-Plus) Functions. R package version 1.3-28.
Lipsitz PJ. A proposed narcotic withdrawal score for use with newborn infants. A pragmatic evaluation of its efficacy. Clin Pediatr. 1975;14:592–4.
Zahorodny W, Rom C, Whitney W, Giddens S, Samuel M, Maichuk G, et al. The Neonatal withdrawal inventory: a simplified score of newborn withdrawal. J Dev Behav Pediatr. 1998;19:89–93.
Green M, Suffet F. The Neonatal narcotic withdrawal index: a device for the improvement of care in the abstinence syndrome. Am J Drug Alcohol Abus. 1981;8:203–13.
Funding
The project described was supported by NIH National Institute on Drug Abuse (R01DA043519) and the NIH National Center for Advancing Translational Sciences (UL1TR001998).
Author information
Authors and Affiliations
Contributions
JSM assisted in the acquisition of data, interpretation of findings, drafted the initial manuscript, and reviewed and revised the manuscript. HB conceptualized the study, coordinated and supervised data collection, and critically revised the manuscript. ML conceptualized the study and critically reviewed the manuscript. PW conceptualized and designed the study, carried out the analyses, interpreted the findings, and critically revised the manuscript. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Miller, J.S., Bada, H.S., Leggas, M. et al. Assessment of the relative clinical utility of shortened Finnegan neonatal abstinence scoring tools. J Perinatol 42, 1051–1057 (2022). https://doi.org/10.1038/s41372-022-01419-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-022-01419-0
- Springer Nature America, Inc.