Abstract
Objective
To correlate arterial umbilical cord gas (aUCG) and infant blood gas with severity of neurological injury.
Study design
Retrospective single-site study of infants evaluated for therapeutic hypothermia. Clinical neurological examination and a validated MRI scoring system were used to assess injury severity.
Results
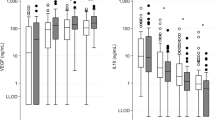
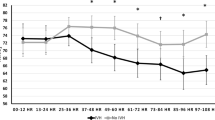
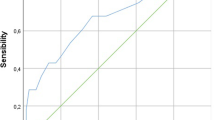
Sixty-eight infants were included. aUCG base deficit (BD) and lactate correlated with infant blood gas counterparts (r = 0.43 and r = 0.56, respectively). aUCG and infant pH did not correlate. Infant blood gas lactate (RADJ2 = 0.40), infant BD (RADJ2 = 0.26), infant pH (RADJ2 = 0.17), aUCG base deficit (RADJ2 = 0.08), and aUCG lactate (RADJ2 = 0.11) were associated with clinical neurological examination severity. aUCG and infant blood gas measures were not correlated with MRI score.
Conclusion
Metabolic measures from initial infant blood gases were most associated with the clinical neurological examination severity and can be used to evaluate hypoxic-ischemic cerebral injury risk.


Similar content being viewed by others
References
Executive summary: Neonatal encephalopathy and neurologic outcome, second edition. Report of the American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Obstet Gynecol. 2014;123:896–901.
Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013;2013:CD003311.
Malin GL, Morris RK, Khan KS. Strength of association between umbilical cord pH and perinatal and long term outcomes: systematic review and meta-analysis. BMJ. 2010;340:c1471.
Westgate J, Garibaldi JM, Greene KR. Umbilical cord blood gas analysis at delivery: a time for quality data. BJOG Int J Obstet Gynaecol. 1994;101:1054–63.
White CRH, Doherty DA, Henderson JJ, Kohan R, Newnham JP, Pennell CE. Benefits of introducing universal umbilical cord blood gas and lactate analysis into an obstetric unit: Universal cord blood gas and lactate analysis. Aust NZ J Obstet Gynaecol. 2010;50:318–28.
Armstrong L, Stenson BJ. Use of umbilical cord blood gas analysis in the assessment of the newborn. Arch Dis Child Fetal Neonatal Ed. 2007;92:F430–4.
ACOG Committee on Obstetric Practice. ACOG Committee Opinion No. 348, November 2006: Umbilical cord blood gas and acid-base analysis. Obstet Gynecol. 2006;108:1319–22.
Cahill A, Mathur A, Smyser C, et al. Neurologic injury in acidemic term infants. Am J Perinatol. 2016;34:668–75.
Casey BM, Goldaber KG, McIntire DD, Leveno KJ. Outcomes among term infants when two-hour postnatal pH is compared with pH at delivery. Am J Obstet Gynecol. 2001;184:447–50.
Andres RL, Saade G, Gilstrap LC, Wilkins I, Witlin A, Zlatnik F, et al. Association between umbilical blood gas parameters and neonatal morbidity and death in neonates with pathologic fetal acidemia. Am J Obstet Gynecol. 1999;181:867–71.
Low JA, Panagiotopoulos C, Derrick EJ. Newborn complications after intrapartum asphyxia with metabolic acidosis in the term fetus. Am J Obstet Gynecol. 1994;170:1081–7.
Knutzen L, Anderson-Knight H, Svirko E, Impey L. Umbilical cord arterial base deficit and arterial pH as predictors of adverse outcomes among term neonates. Int J Gynaecol Obstet. 2018;142:66–70.
Tuuli MG, Stout MJ, Shanks A, Odibo AO, Macones GA, Cahill AG. Umbilical cord arterial lactate compared with pH for predicting neonatal morbidity at term. Obstet Gynecol. 2014;124:756–61.
Wiberg N, Källén K, Herbst A, Olofsson P. Relation between umbilical cord blood pH, base deficit, lactate, 5-minute Apgar score and development of hypoxic ischemic encephalopathy. Acta Obstet Gynecol Scand. 2010;89:1263–9.
Ambalavanan N, Carlo WA, Shankaran S, Bann CM, Emrich SL, Higgins RD, et al. Predicting outcomes of neonates diagnosed with hypoxemic-ischemic encephalopathy. Pediatrics. 2006;118:2084–93.
Vesoulis ZA, Liao SM, Rao R, Trivedi SB, Cahill AG, Mathur AM. Re-examining the arterial cord blood gas pH screening criteria in neonatal encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2018;103:F377–82.
Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976;33:696–705.
Thompson C, Puterman A, Linley L, Hann F, Elst C, Molteno C, et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr. 1997;86:757–61.
Miller SP, Latal B, Clark H, Barnwell A, Glidden D, Barkovich AJ, et al. Clinical signs predict 30-month neurodevelopmental outcome after neonatal encephalopathy. Am J Obstet Gynecol. 2004;190:93–9.
Benninger KL, Inder TE, Goodman AM, Cotten CM, Nordli DR, Shah TA, et al. Perspectives from the Society for Pediatric Research. Neonatal encephalopathy clinical trials: develo** the future. Pediatr Res. 2020. http://www.nature.com/articles/s41390-020-0859-9.
Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–84.
Shankaran S, Laptook AR, Tyson JE, Ehrenkranz RA, Bann CM, Das A, et al. Evolution of encephalopathy during whole body hypothermia for neonatal hypoxic-ischemic encephalopathy. J Pediatr. 2012;160:567–72.e3.
Inder TE, Volpe JJ. Chapter 20—Hypoxic-ischemic injury in the term infant: clinical-neurological features, diagnosis, imaging, prognosis, therapy. In: Volpe JJ, Inder TE, Darras BT, de Vries LS, du Plessis AJ, Neil JJ, et al., editors. Volpe’s neurology of the newborn. 6th ed. Elsevier; 2018. p. 510–563.e15.
Perez JMR, Golombek SG, Sola A, et al. Clinical hypoxic-ischemic encephalopathy score of the Iberoamerican Society of Neonatology (Siben): a new proposal for diagnosis and management. Rev Assoc Med Bras. 2017;63:64–9.
Perez JMR, Golombek SG, Alpan G, Sola A. Using a novel laminar flow unit provided effective total body hypothermia for neonatal hypoxic encephalopathy. Acta Paediatr. 2015;104:e483–8.
Groenendaal F, de Vries LS. Fifty years of brain imaging in neonatal encephalopathy following perinatal asphyxia. Pediatr Res. 2017;81:150–5.
Weeke LC, Groenendaal F, Mudigonda K, Blennow M, Lequin MH, Meiners LC, et al. A novel magnetic resonance imaging score predicts neurodevelopmental outcome after perinatal asphyxia and therapeutic hypothermia. J Pediatr. 2018;192:33–40.e2.
Gunn AJ, Wyatt JS, Whitelaw A, Barks J, Azzopardi D, Ballard R, et al. Therapeutic hypothermia changes the prognostic value of clinical evaluation of neonatal encephalopathy. J Pediatr. 2008;152:55–58.e1.https://doi.org/10.1016/C2010-0-68825-0.
Kaufman SA, Miller SP, Ferriero DM, Glidden DH, Barkovich AJ, Partridge JC. Encephalopathy as a predictor of magnetic resonance imaging abnormalities in asphyxiated newborns. Pediatr Neurol. 2003;28:342–6.
Walsh BH, Neil J, Morey J, Yang E, Silvera MV, Inder TE, et al. The frequency and severity of magnetic resonance imaging abnormalities in infants with mild neonatal encephalopathy. J Pediatr. 2017;187:26–33.e1.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81.
Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125:e214–24.
Holzmann M, Cnattingius S, Nordström L. Lactate production as a response to intrapartum hypoxia in the growth-restricted fetus: hypoxia and lactate production in the IUGR fetus. BJOG Int J Obstet Gynaecol. 2012;119:1265–9.
Milsom I, Ladfors L, Thiringer K, Niklasson A, Odeback A, Thornberg E. Influence of maternal, obstetric and fetal risk factors on the prevalence of birth asphyxia at term in a Swedish urban population. Acta Obstet Gynecol Scand. 2002;81:909–17.
Inder TE, Volpe JJ. Chapter 17—Intrauterine, Intrapartum Assessments in the Term Infant. In: Volpe JJ, Inder TE, Darras BT, de Vries LS, du Plessis AJ, Neil JJ, et al., editors. Volpe’s neurology of the newborn. 6th ed. Elsevier; 2018. p. 458–483.e458.
Cahill AG, Macones GA, Smyser CD, López JD, Inder TE, Mathur AM. Umbilical artery lactate correlates with brain lactate in term infants. Am J Perinatol. 2017;34:535–40.
Low JA, Lindsay BG, Derrick EJ. Threshold of metabolic acidosis associated with newborn complications. Am J Obstet Gynecol. 1997;177:1391–4.
Fauchère J-C, Bauschatz A, Arlettaz R, Zimmermann-Bär U, Bucher H. Agreement between capillary and arterial lactate in the newborn. Acta Paediatr. 2007;91:78–81.
Al Balushi A, Guilbault M-P, Wintermark P. Secondary increase of lactate levels in asphyxiated newborns during hypothermia treatment: reflect of suboptimal hemodynamics (A case series and review of the literature). Am J Perinatol Rep. 2015;06:e48–58.
Cousineau J, Anctil S, Carceller A, Gonthier M, Delvin EE. Neonate capillary blood gas reference values. Clin Biochem. 2005;38:905–7.
Acknowledgements
We thank Elizabeth Singh, Kirsten Thiim, and Song Ha Lee, our research assistants at BWH, for their assistance in collecting data.
Funding
This study was undertaken with local departmental funding support.
Author information
Authors and Affiliations
Contributions
KS: had substantial contributions to conception and design, acquisition of data, analysis and interpretation of data, drafted the article, and had final approval of the version to be published. KJS: had substantial contributions to conception, acquisition of data, analysis and interpretation of data, contributed to revising it critically for important intellectual content, and had final approval of the version to be published. MEl-D: had substantial contributions to conception and design, acquisition of data, interpretation of data, contributed to revising it critically for important intellectual content, and had final approval of the version to be published. ES: had substantial contributions to acquisition of data, interpretation of data, contributed to revising it critically for important intellectual content, and had final approval of the version to be published. EY: had substantial contributions to acquisition of data, interpretation of data, contributed to revising it critically for important intellectual content, and had final approval of the version to be published. BHW: had substantial contributions to conception and design, acquisition of data, interpretation of data, contributed to revising it critically for important intellectual content, and had final approval of the version to be published. JNR: had substantial contributions to conception and interpretation of data, contributed to revising it critically for important intellectual content, and had final approval of the version to be published. SC: had substantial contributions to acquisition of data, analysis and interpretation of data, contributed to revising it critically for important intellectual content, and had final approval of the version to be published. JJV: had substantial contributions to conception and interpretation of data, contributed to revising it critically for important intellectual content, and had final approval of the version to be published. TEI: had substantial contributions to conception and design, acquisition and interpretation of data, contributed to revising it critically for important intellectual content, and had final approval of the version to be published.
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sakpichaisakul, K., Supapannachart, K.J., El-DIb, M. et al. Blood gas measures as predictors for neonatal encephalopathy severity. J Perinatol 41, 2261–2269 (2021). https://doi.org/10.1038/s41372-021-01075-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-021-01075-w
- Springer Nature America, Inc.