Abstract
Oncogene-induced senescence (OIS) or apoptosis through the DNA-damage response is an important barrier of tumorigenesis. Overcoming this barrier leads to abnormal cell proliferation, genomic instability, and cellular transformation, and finally allows cancers to develop. However, it remains unclear how the OIS barrier is overcome. Here, we show that the E3 ubiquitin ligase WD repeat and SOCS box-containing protein 1 (WSB1) plays a role in overcoming OIS. WSB1 expression in primary cells helps the bypass of OIS, leading to abnormal proliferation and cellular transformation. Mechanistically, WSB1 promotes ATM ubiquitination, resulting in ATM degradation and the escape from OIS. Furthermore, we identify CDKs as the upstream kinase of WSB1. CDK-mediated phosphorylation activates WSB1 by promoting its monomerization. In human cancer tissue and in vitro models, WSB1-induced ATM degradation is an early event during tumorigenic progression. We suggest that WSB1 is one of the key players of early oncogenic events through ATM degradation and destruction of the tumorigenesis barrier. Our work establishes an important mechanism of cancer development and progression in premalignant lesions.
Similar content being viewed by others
Introduction
Oncogene-induced senescence (OIS) is an important cellular response for protection against cancer development1,2,3,42, HIPK227, and pVHL34 have been identified. Therefore, we hypothesized that ATM is ubiquitinated by WSB1, which results in its downergulation. To test this hypothesis, we first examined the ability of WSB1 to interact with ATM. The association of endogenous ATM with WSB1 was detected via co-immunoprecipitation, and this binding was increased after MG132 treatment, which increased both ATM and WSB1 levels (Figure 3A). To identify the regions of ATM involved in binding WSB1, we performed glutathione S-transferase (GST) pull-down experiments using 8 overlap** GST-ATM fragments spanning full-length ATM43. WSB1 bound strongly to GST-ATM-5 (a.a. 1 690-2 120) and weakly to GST-ATM-8 (a.a. 2 680-3 056, encompassing the kinase domain; Figure 3B). We further determined whether WSB1 could regulate ATM ubiquitination. We found that WSB1 overexpression increased ATM ubiquitination (Figure 3C). In addition, in cells depleted of WSB1, ATM stability increased (Figure 3D). Furthermore, p53 stability, which is regulated by ATM, was also increased in WSB1-depleted cells. However, we did not find WSB1 to regulate ATR or DNA-PK stability, nor did it interact with ATR or regulate ATR expression (Figure 3D-3F). These results suggest that WSB1 regulates ATM levels through the ubiquitin-proteasome pathway.
WSB1 regulates ATM ubiquitination and degradation. (A) Co-immunoprecipitation (Co-IP) of endogenous ATM and WSB1 from extracts of HEK 293T cells. (B) Purified WSB1 were incubated with GST or equal amounts of GST-ATM fragments coupled to GSH sepharose. Proteins retained on sepharose were then blotted with the indicated antibodies. (C) Cells were transfected with the indicated constructs and then treated with MG132. Ubiquitinated proteins were pulled-down under denaturing conditions by Ni-NTA agarose and analyzed by immunoblot. (D) HEK 293T cells stably expressing control shRNA or WSB1 shRNA (#1 and #2) were treated with CHX (0.1 mg/ml) and harvested at the indicated times. Cell lysates were then blotted with the indicated antibodies (left). Data (left) were quantified and normalized relative to the β-actin level using ImageJ program (right). **P < 0.01 and ***P < 0.001 versus control cells by one-way ANOVA. (E) Co-IP of WSB1 and ATM or ATR from extracts of HEK 293T cells. (F) Cells were transfected with WSB1 shRNAs and ATM or ATR levels were examined by immunoblot. (G, H) Cells were transfected with the indicated constructs and then treated with MG132. Ubiquitinated proteins were pulled-down under denaturing conditions by Ni-NTA agarose and analyzed by immunoblot. (I) ATM KO MEFs were transfected with the indicated constructs and ATM levels were then examined by immunoblot (top). Quantification of ATM protein expression levels are shown in top panel (bottom). *P < 0.05 and ***P < 0.001 versus control cells by one-way ANOVA. (J) Cells were infected (or transfected by electroporation) with the indicated viruses (or constructs) and analyzed for senescence (top, SA-β-gal staining; bottom, quantification of senescent or transformed cells).
As some ATM ubiquitination sites were revealed by mass spectrometry analysis (http://www.phosphosite.org), we analyzed whether these sites were ubiquitinated by WSB1. Of them, mutation of either K1323 or K2025 to arginine (R) substantially reduced ATM ubiquitination (Figure 3G), suggesting that these two sites might be major sites of ubiquitination mediated by WSB1. As shown in Figure 3H, double mutation of both K1323 and K2025 residues (2KR) largely abolished ATM ubiquitination induced by WSB1 overexpression. We therefore predicted that the 2KR mutant would be resistant to WSB1-mediated downregulation. To test this hypothesis, we stably expressed Flag-tagged WT ATM or ATM mutants (K1323R, K2025R, K1323R/K2025R) in ATM−/− immortal MEFs (Figure 3I). The level of WT ATM was dramatically decreased by WSB1 overexpression. The expression of single KR mutants of ATM could also be decreased by WSB1 expression, although to a lesser extent than WT ATM. Importantly, the expression of 2KR was not affected by WSB1 expression (Figure 3I). We next tested whether reintroducing 2KR, but not WT ATM can confer resistance to WSB1-dependent escape of OIS. Both control cells and MEFs expressing WT ATM could be transformed by H-RasV12 (Figure 3J). However, cells expressing 2KR were senescent and resistant to transformation by H-Ras. Furthermore, WSB1 knockdown inhibited cell transformation induced by H-RasV12 in cells expressing WT ATM, and had no additional effect in cells expressing the 2KR mutant. All of the above results indicated that K1323 and K2025 are major ATM ubiquitination sites regulated by WSB1, and ubiquitination of these residues leads to ATM degradation.
The SOCS domain of WSB1 is required for ATM interaction and ubiquitination
We next determined how WSB1 interacts with ATM. WSB1 contains several WD40 repeats and a SOCS domain42. WD40 repeats participate in various cellular functions, and the SOCS box is involved in protein degradation by the Elongin-Cullin-SOCS-box protein ubiquitin ligase complex44,45,46. To determine the region of WSB1 that is required for its interaction with ATM, we transfected Myc-tagged WSB1-deletion mutants into HEK 293T cells and performed co-immunoprecipitation. We found that the C-terminal WD40 repeats (WD 6-7) and the SOCS domain of WSB1 were both required and sufficient for ATM interaction (Figure 4A and 4B). We further found that downregulation of ATM by WSB1 was dependent on the SOCS domain (Figure 4A and 4C). Consistent with these results, we found that ubiquitination of ATM by WSB1 required the SOCS domain (Figure 4D). In addition, ATM was more stable in cells expressing the WSB1 mutant lacking the SOCS domain (Figure 4E). Together, these results indicate that WSB1 interacts with ATM via its WD40 repeats and SOCS domain and regulates ATM ubiquitination and degradation through the SOCS domain.
WSB1 interacts with ATM via its WD40 repeats and SOCS domain and regulates ATM ubiquitination through the SOCS domain. (A) Diagrams of WT WSB1 and corresponding deletion mutants (ΔWD 1-3, ΔWD 1-5, ΔSOCS, and Δ6-SOCS) used in Co-IP experiments with ATM. Plus symbols indicate binding ability between different WSB1 mutants with ATM (left) or the degradation strength of the WSB1 mutants on ATM stability (right). (B) Cells were transfected with indicated plasmids and then treated with MG132. Cells were collected for immunoprecipitation (IP)-immunoblot analysis. (C) Cells were transfected with indicated plasmids and the ATM levels were examined by immunoblot. (D) Cells were transfected with the indicated constructs and then treated with MG132. Ubiquitinated proteins were pulled-down under denaturing conditions by Ni-NTA agarose and analyzed by immunoblot. S.E. indicates short exposure; L.E., long exposure. (E) Cells were treated with CHX (0.1 mg/ml) and harvested for different hours. ATM levels were then examined by immunoblot (left). Right panel shows quantification of ATM levels. The results represent the means (± SE) of three independent experiments performed in triplicate. **P < 0.01 and ***P < 0.001 versus control cells by one-way ANOVA. Data (left) were quantified and normalized relative to the β-actin level using ImageJ program (right). (F, G) Primary MEFs were infected with the indicated constructs and were analyzed for senescence by SA-β-gal staining assay (F) and cell-proliferation assay (G). Scale bar, 10 μm. Yellow arrows indicate SA-β-gal stained cells; red arrows, transformed cells; white arrow, death cells. The results represent the means (± SE) of three independent experiments performed in triplicate. **P < 0.01 and ***P < 0.001 versus control cells by one-way ANOVA. (H) Cells as in F were collected for immunoblot analysis. (I, J) Primary MEF cells as in F were applied on 3-D organoid cultures system. Schematic of the experiments (I) and sphere number (J) in 3-D organoid culture. ****P < 0.0001 versus control cells by one-way ANOVA. (K) IMR-90 ER:Ras-inducible cells were infected with indicated viruses, and H-Ras was inducted by 4-OHT. Cells were analyzed for 'cytokine' mRNAs by real-time PCR analysis.
We reasoned that WSB1-induced ubiquitination and degradation of ATM could facilitate cellular transformation by oncogene through the suppression of DDR. Given the important function of the WSB1 SOCS domain in mediating ATM degradation, we investigated how the WSB1 mutant lacking the SOCS domain affects OIS. We infected MEFs with viruses encoding both H-RasV12 and WSB1 (WT or ΔSOCS) and examined OIS and cellular transformation. Consistent with Figure 1, co-expression of oncogenic Ras and WSB1 promoted the escape from OIS and cell transformation (Figure 4F and 4G). Deletion of the SOCS domain abolished the ability of WSB1 to promote the escape from OIS, cell transformation, and abnormal cell proliferation (Figure 4F and 4G). ATM levels were decreased in cells expressing WT WSB1 but not in cells expressing mutant WSB1 (ΔSOCS; Figure 4H). In 3-D culture systems, we obtained similar results. WT WSB1, but not ΔSOCS, together with H-RasV12 expression, promoted sphere formation (Figure 4I and 4J). Since senescence-associated secretion phenotype (SASP) is a major characteristic of OIS3,47, we next examined the impact of WSB1 on SASP. We found that overexpression of WT WSB1 suppressed the expression of key SASP factors such as IL-6, IL-8, and IL-1β during OIS after H-RasV12 induction in IMR-90 ER:Ras cells (Figure 4K). However, WSB1 mutant lacking the SOCS domain did not affect SASP gene expression. These results together with Figure 2 suggest that one major mechanism by which WSB1 promotes the escape from OIS thus leading to cellular transformation is through inducing ATM ubiquitination and its subsequent degradation.
WSB1 phosphorylation by CDKs is required for its monomerization and activity
We next sought to determine the molecular mechanisms that regulate WSB1. For several E3 ligases, such as MDM2 and TRAF6, dimerization or oligomerization is involved in the regulation of activity48,43. WSB1 and ATM mutations were generated by site-directed mutagenesis (Stratagene).
Transient transfection, viral infection, and stable transduction
shRNAs or viral vectors were infected using Lipofectamine 2000 reagent (Invitrogen). Human WSB1 and mouse WSB1 shRNAs were obtained from Sigma-Aldrich and Open Biosystems.
WSB1 shRNA (human): Open Biosystems
5′-GCTGTTGACAGTGAGCGCGGAGTTTCTCTCGTATCGTATTAGTGAAGCCACAGATGTAATACGATACGAGAGAAACTCCATGCCTACTGCCTCGGA-3′
5′-GCTGTTGACAGTGAGCGCGCTGTAAAGTGCAAGGAAATTTAGTGAAGCCACAGATGTAAATTTCCTTGCACTTTACAGCATGCCTACTGCCTCGGA-3′
WSB1 shRNA (Mouse): Sigma-Aldrich
5′-ACATGAGCTGCTGCTATATAT-3′
5′-GCTTACTCCTTGTATCAGCTT-3′
ATM shRNA (Mouse): Sigma-Aldrich
5′-CCGTGGAGATTTCTCAATCTT-3′
5′-CCTCCAATTCTTCAGCGTAAT-3′
5′-GCTGAGACAAATAATGTCTTT-3′
5′-CGATGGAAGTTATGCGGAGTT-3′
5′-CCACCATATTTGGACAGGAAT-3′
For transient overexpression studies, DNA plasmids were transfected using Lipofectamine 2000 reagent (Invitrogen). Stable overexpression and silencing were obtained by transducing IMR-90 and HEK 293T cells with retroviral or lentiviral vectors. The efficiency of knock-down or overexpression was controlled by Western blotting. Infected cells were selected with 2 μg/ml puromycin (Sigma-Aldrich).
Colony formation and SA-β-gal
For colony-formation or foci assay, early-passage MEFs (passage 5) cells were plated at low density into 60-mm cell culture plates. When sufficient colonies were visible, typically after 2-3 weeks, cells were washed twice in PBS before fixing in ice-cold 70% methanol for 30 min and then stained by 0.2% crystal violet for 0.5-1 h. The following day, cells were rinsed in PBS and air dried. For SA-β-Gal, MEFs were fixed in 2% formaldehyde/0.2% glutaraldehyde in PBS for 10 min and stained for SA-β-Gal according to manufacturer's instructions (Cell Signaling) overnight at 37 °C.
Protein stabilization analysis
For protein stabilization analysis, HeLa cells were transfected with the indicated constructs. After transfection for 48 h, cells were treated with CHX (20 μg/ml). The cell lysates were prepared and analyzed by Western blot analysis. After CHX treatment, endogenous or exogenous Myc levels were quantified by densitometric scanning in the image J program.
Co-immunoprecipitation assays, immunoblotting, and antibodies
To study endogenous WSB1/ATM binding, the cells were treated with or without 10 μM MG132 for 2 h. One confluent 15-cm dish of HEK 293T cells was lysed by sonication in NETN buffer (20 mM Tris-HCl, pH 8.0, 100 mM NaCl2, 1 mM EDTA, and 0.5% Nonidet P-40) containing 50 mM b-glycerophosphate,10 mM NaF, and 1 mg/ml each of pepstatin A and aprotinin, freshly supplemented with a protease inhibitor cocktail (Roche). Prior to immunoprecipitation, protein-A-bound agarose beads were incubated overnight with ATM (Abcam, ab32420), p-ATM (Abcam, ab81292), WSB1 (Abcam, ab68953; Sigma, HPA003293; Proteintech, 1166-1-AP), Ki-67 (Abcam, ab15580), p53 (DO-1; SantaCruz, sc-126), p16 (Abcam, ab51243), and ATR antibodies in PBS with 5% BSA at 4 °C. We used WSB1 antibody of ab68953 for most data (HPA003293 for IHC). We then added to the extracts before immunoprecipitation with protein-A sepharose at 4 °C for 4 h. After three washings in a binding buffer, co-purified proteins were analyzed by Western blotting. For the map** of the ATM-interacting domain of WSB1, HEK 293T cells were transfected with a 1 μg/10 cm dish of either wild-type or mutant forms of Myc-WSB1 and harvested in the presence of 10 μM MG132. Cell extract was then subjected to immunoprecipitation using anti-Myc. For removing heavy-chain, heavy- or light-chain-specific anti-mouse and anti-rabbit IgG, secondary antibodies were obtained from Jackson Immunoresearch. Anti-α-tubulin, Myc, and HA mouse antibodies were purchased from Sigma. To study WSB1 dimerization and/or monomerizaton, reduced samples were prepared with a reducing sample buffer containing β-ME and SDS, and then boiled. Non-reducing samples were put in the sample buffer without β-ME, which were not boiled. To study WSB1 phosphorylation by CDK2, we used an anti-Phospho-(Thr) MAPK/CDK substrate (Cell Signaling). Antibodies used in the other studies included the following: CDK1 (Abcam, ab32384), CDK2 (Abcam, ab64669), and CDK4 (Abcam, ab137675).
In vivo ubiquitination assays
For in vivo ubiquitination, including domain map** of WSB1, cells were transfected with ubiquitin-His plasmid together with Myc or Myc-WSB1, followed by treatment with MG 132 (10 μM). Forty-eight hours post-transfection, cells were lysed by Urea lysis buffer (8 M urea, 0.1 M Na2HPO4, 0.1 M Tris/HCl (pH 8.0), 0.05% Tween 20, and 0.01 M imidazole). After centrifugation, the supernatants were collected and incubated with 20 ml Ni-NTA agarose beads (Qiagen) for 4 h at 4 °C. The precipitates were washed three times with Urea wash buffer (8 M urea, 0.1 M Na2HPO4, 0.1 M Tris/HCl (pH 8.0), 0.05% Tween 20, and 0.02 M imidazole) and Native wash buffer (0.1 M Na2HPO4, 0.1 M Tris/HCl (pH 8.0), 0.05% Tween 20, and 0.02 M imidazole), boiled with SDS loading buffer, and then subjected to SDS-PAGE followed by immunoblot analysis.
In vitro binding assay
GST fusion proteins were prepared following standard protocol79. For in vitro biding assays, ATM (8 deleted mutants) GST fusion proteins bound to the GSH Sepharose were incubated with cell lysates. After washing, the bound proteins were separated by SDS-PAGE and immunoblotted with indicated antibodies.
Immunofluorescence
For immunofluorescence staining, MEF and IMR-90 cells were plated on glass cover slips and infected with the indicated constructs. Cells were then fixed in 3.7% paraformaldehyde for 10 min at room temperature and stained using standard protocols. Immunofluorescence images were taken using fluorescent microscopy (Nikon Microscope, Melville, NY, USA).
Proliferation and viability analysis
For proliferation staining, MEFs were plated on glass cover slips and infected with the indicated constructs. Cells were then fixed and stained with Ki-67 antibody. For viability staining, MEF cells were infected with the indicated constructs and were seeded in six-well plates and cultured for 15 days. Cells were then washed three times with PBS and stained with 0.2% crystal violet for 30 min before cell counting under microscopy.
In vitro kinase assay
HEK 293T cells were transfected with Flag-CDK2 and V5-CyclinE. Forty-eight hours later, the cells were collected and the cell lysates were subjected to immunoprecipitated anti-Flag antibody. The protein conjugated to the beads were eluted with Flag peptides and subjected to in vitro kinase assay, or the active CDK2/CyclinE protein from Millipore was used as kinase in the assay. GST and GST-WSB1 expressed in bacteria were purified and subjected to kinase assay as substrates. The phosphorylation of WSB1 by CDK2 was examined using an anti-CDK substrate antibody.
Reverse transcription-PCR of cDNA
RNA preparation and cDNA were described previously80. The following primers were used:
WSB1 forward 5′-TCTCCTGACTCTTCTATGCTGTGT-3′
reverse 5′-CATGGTGTATTTATCCATATTCCAAA-3′
ATM forward 5′-GTGTTCTGAAATTGTGAACCATGAGTCTAGT-3′
reverse 5′-TGGTATCTTCATTAAAAACCTGGTGACAGA-3′
forward 5′-GATGGAGAAAGTAGTGATGAGC-3′
reverse 5′-AGTCACCAGATTTCCATATTCTC-3′
The β-actin sequence was described previously80.
Electroporation
For complementation experiments, Vector, Flag-ATM-WT, or Flag-ATM mutant plasmids (K1109R, K1114R, K1323R, K1572R, K2025R, and 2KR), Flag-CDK1, Flag-CDK2, Flag-CDK4, or HA-WSB1 were electroporated into ATM KO MEFs using GenePulser Xcell (Bio-Rad). Pellet cells were resuspended in 10 ml of DMEM. The cells were either re-suspended (107) in 400 μl of transfection serum-free medium and 10-40 μg of plasmid DNA added or re-suspended to the cells (5 × 107) in 400 μl of transfection serum-free medium and 40 μg of plasmid DNA added. Electroporation used the following settings: square wave pulse, 200 V, 30 ms, and 4 mm cuvette. The cuvette was placed on ice for 5-10 min. Cells were transferred to a 60-mm dish and 4 ml of media added (15% serum; without antibiotics and selective agent). Cells were then incubated overnight and media was changed at the next day.
Mouse xenograft tumor model
For MEF xenograft experiments, cells were infected (or transfected) with the indicated constructs (by electroporation) and injected into nude mice. Equal numbers (1 × 106) of infected MEF cells expressing luciferase mixed at a 1:1 dilution with Matrigel (Collaborative Research) were implanted in the backs of athymic nude mice. For CDK-related xenograft experiments, mice were treated with CDKIs, such as RO-3306 (CDK1 inhibitor), SNS-032 (BMS-387032, CDK1 inhibitor) and PD0332991 (CDK4 inhibitor). Tumor growth was monitored using calipers and visualized with a bioluminescence-based IVIS system (Caliper LifeScience). Tumor growth was measured using a Vernier caliper at the indicated times after injection, and the tumor volume was calculated as length × width × height. Tumor size was monitored by measuring mice two times a week. When tumors reached 2 cm in diameter, mice were euthanized.
Immunohistochemistry
All of step for IHC were prepared following standard protocol. Briefly, immunohistochemical cytokeratin staining was performed on formalin-fixed, paraffin-embedded tissue using an indirect immunoperoxidase technique. Sections mounted on silanized slides were dewaxed in xylene, dehydrated in ethanol, boiled in 0.01 M citrate buffer (pH 6.0) for 20 min in a microwave oven and then incubated with 3 % hydrogen peroxide for 5 min. After washing with PBS, the slides were incubated in 10 % normal BSA for 5 min, followed by incubation for 45 min with monoclonal mouse anti-human WSB1 (1:200, Sigma-Aldrich) and anti-ATM (1:100, Abcam). After washing, sections were incubated with labeled polymer (Bond Polymer Refine Detection) and diaminobenzidine. The sections were then counterstained with hematoxylin, dehydrated, cleared, and mounted.
Statistical analysis
Each assay was performed in triplicate and independently repeated at least three times. The results are presented as mean ± SEM. Statistical analyses were performed using GraphPad Prism software (version 4.02; GraphPad Software, San Diego, CA, USA). One-way analysis of variance followed by t-testing was used to compare the results. A difference was considered significant if P < 0.05. Statistical significance was defined as P < 0.05 (*), P < 0.01 (**), P < 0.001 (***), and P < 0.0001 (****).
Author Contributions
SBL, JJK, and ZL designed and performed most of the experiments, analyzed data, and prepared the manuscript as lead authors. SYY, SHK, SAH, JML, and SYT provided clinical samples and analyzed data. PY and BG performed animal experiments. ZL designed and supervised the study.
Competing Financial Interests
The authors declare no competing financial interests.
References
Lowe SW, Cepero E, Evan G . Intrinsic tumour suppression. Nature 2004; 432:307–315.
Collado M, Gil J, Efeyan A, et al. Tumour biology: senescence in premalignant tumours. Nature 2005; 436:642.
Munoz-Espin D, Serrano M . Cellular senescence: from physiology to pathology. Nat Rew Mol Cell Biol 2014; 15:482–496.
Xu Y, Li N, **ang R, Sun P . Emerging roles of the p38 MAPK and PI3K/AKT/mTOR pathways in oncogene-induced senescence. Trends Biochem Sci 2014; 39:268–276.
Halazonetis TD, Gorgoulis VG, Bartek J . An oncogene-induced DNA damage model for cancer development. Science 2008; 319:1352–1355.
Venkitaraman AR . Medicine: aborting the birth of cancer. Nature 2005; 434:829–830.
Bartek J, Bartkova J, Lukas J . DNA damage signalling guards against activated oncogenes and tumour progression. Oncogene 2007; 26:7773–7779.
Hills SA, Diffley JF . DNA replication and oncogene-induced replicative stress. Curr Biol 2014; 24:R435–R444.
Reddy JP, Peddibhotla S, Bu W, et al. Defining the ATM-mediated barrier to tumorigenesis in somatic mammary cells following ErbB2 activation. Proc Natl Acad Sci USA 2010; 107:3728–3733.
Sulli G, Di Micco R, d'Adda di Fagagna F . Crosstalk between chromatin state and DNA damage response in cellular senescence and cancer. Nat Rev Cancer 2012; 12:709–720.
Aird KM, Worth AJ, Snyder NW, et al. ATM couples replication stress and metabolic reprogramming during cellular senescence. Cell Rep 2015; 11:893–901.
Bartkova J, Horejsi Z, Koed K, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 2005; 434:864–870.
Campisi J . Cellular senescence: putting the paradoxes in perspective. Curr Opin Genet Dev 2011; 21:107–112.
Gorgoulis VG, Vassiliou LV, Karakaidos P, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 2005; 434:907–913.
Reimann M, Loddenkemper C, Rudolph C, et al. The Myc-evoked DNA damage response accounts for treatment resistance in primary lymphomas in vivo. Blood 2007; 110:2996–3004.
Pusapati RV, Rounbehler RJ, Hong S, et al. ATM promotes apoptosis and suppresses tumorigenesis in response to Myc. Proc Natl Acad Sci USA 2006; 103:1446–1451.
Bartkova J, Rezaei N, Liontos M, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 2006; 444:633–637.
Di Micco R, Fumagalli M, Cicalese A, et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 2006; 444:638–642.
Mallette FA, Gaumont-Leclerc MF, Ferbeyre G . The DNA damage signaling pathway is a critical mediator of oncogene-induced senescence. Genes Dev 2007; 21:43–48.
Aird KM, Zhang R . ATM in senescence. Oncotarget 2015; 6:14729–14730.
Suram A, Kaplunov J, Patel PL, et al. Oncogene-induced telomere dysfunction enforces cellular senescence in human cancer precursor lesions. EMBO J 2012; 31:2839–2851.
Shiloh Y, Ziv Y . The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat Rev Mol Cell Biol 2013; 14:197–210.
Kastan MB, Lim DS . The many substrates and functions of ATM. Nat Rev Mol Cell Biol 2000; 1:179–186.
Silins I, Finnberg N, Stahl A, Hogberg J, Stenius U . Reduced ATM kinase activity and an attenuated p53 response to DNA damage in carcinogen-induced preneoplastic hepatic lesions in the rat. Carcinogenesis 2001; 22:2023–2031.
Angele S, Jones C, Reis Filho JS, et al. Expression of ATM, p53, and the MRE11-Rad50-NBS1 complex in myoepithelial cells from benign and malignant proliferations of the breast. J Clin Pathol 2004; 57:1179–1184.
Feng X, Li H, Dean M, et al. Low ATM protein expression in malignant tumor as well as cancer-associated stroma are independent prognostic factors in a retrospective study of early-stage hormone-negative breast cancer. Breast Cancer Res 2015; 17:65.
Choi DW, Seo YM, Kim EA, et al. Ubiquitination and degradation of homeodomain-interacting protein kinase 2 by WD40 repeat/SOCS box protein WSB-1. J Biol Chem 2008; 283:4682–4689.
Vasiliauskas D, Hancock S, Stern CD . SWiP-1: novel SOCS box containing WD-protein regulated by signalling centres and by Shh during development. Mech Dev 1999; 82:79–94.
Rhodes DR, Chinnaiyan AM . Integrative analysis of the cancer transcriptome. Nat Genet 2005; 37 Suppl:S31–37.
Benita Y, Kikuchi H, Smith AD, Zhang MQ, Chung DC, Xavier RJ . An integrative genomics approach identifies Hypoxia Inducible Factor-1 (HIF-1)-target genes that form the core response to hypoxia. Nucleic Acids Res 2009; 37:4587–4602.
Archange C, Nowak J, Garcia S, et al. The WSB1 gene is involved in pancreatic cancer progression. PloS One 2008; 3:e2475.
Silva AS, Wood SH, van Dam S, Berres S, McArdle A, de Magalhaes JP . Gathering insights on disease etiology from gene expression profiles of healthy tissues. Bioinformatics 2011; 27:3300–3305.
Tong Y, Li QG, **ng TY, Zhang M, Zhang JJ, **a Q . HIF1 regulates WSB-1 expression to promote hypoxia-induced chemoresistance in hepatocellular carcinoma cells. FEBS Lett 2013; 587:2530–2535.
Kim JJ, Lee SB, Jang J, et al. WSB1 promotes tumor metastasis by inducing pVHL degradation. Genes Dev 2015; 29:2244–2257.
Evangelou K, Bartkova J, Kotsinas A, et al. The DNA damage checkpoint precedes activation of ARF in response to escalating oncogenic stress during tumorigenesis. Cell Death Differ 2013; 20:1485–1497.
Pampaloni F, Reynaud EG, Stelzer EH . The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol 2007; 8:839–845.
Jechlinger M . Organotypic culture of untransformed and tumorigenic primary mammary epithelial cells. Cold Spring Harb Protoc 2015; 2015:pdb prot078295.
Di Micco R, Sulli G, Dobreva M, et al. Interplay between oncogene-induced DNA damage response and heterochromatin in senescence and cancer. Nat Cell Biol 2011; 13:292–302.
Luo X, Suzuki M, Ghandhi SA, Amundson SA, Boothman DA . ATM regulates insulin-like growth factor 1-secretory clusterin (IGF-1-sCLU) expression that protects cells against senescence. PloS One 2014; 9:e99983.
Abdel-Fatah TM, Arora A, Alsubhi N, et al. Clinicopathological significance of ATM-Chk2 expression in sporadic breast cancers: a comprehensive analysis in large cohorts. Neoplasia 2014; 16:982–991.
Bueno RC, Canevari RA, Villacis RA, et al. ATM down-regulation is associated with poor prognosis in sporadic breast carcinomas. Ann Oncol 2014; 25:69–75.
Dentice M, Bandyopadhyay A, Gereben B, et al. The Hedgehog-inducible ubiquitin ligase subunit WSB-1 modulates thyroid hormone activation and PTHrP secretion in the develo** growth plate. Nat Cell Biol 2005; 7:698–705.
Takai H, Wang RC, Takai KK, Yang H, de Lange T . Tel2 regulates the stability of PI3K-related protein kinases. Cell 2007; 131:1248–1259.
Xu C, Min J . Structure and function of WD40 domain proteins. Protein Cell 2011; 2:202–214.
Zhang JG, Farley A, Nicholson SE, et al. The conserved SOCS box motif in suppressors of cytokine signaling binds to elongins B and C and may couple bound proteins to proteasomal degradation. Proc Natl Acad Sci USA 1999; 96:2071–2076.
Pozzebon ME, Varadaraj A, Mattoscio D, et al. BC-box protein domain-related mechanism for VHL protein degradation. Proc Natl Acad Sci USA 2013; 110:18168–18173.
Coppe JP, Desprez PY, Krtolica A, Campisi J . The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 2010; 5:99–118.
Yin Q, Lin SC, Lamothe B, et al. E2 interaction and dimerization in the crystal structure of TRAF6. Nat Struct Mol Biol 2009; 16:658–666.
Liu T, Zhang H, **ong J, Yi S, Gu L, Zhou M . Inhibition of MDM2 homodimerization by XIAP IRES stabilizes MDM2, influencing cancer cell survival. Mol Cancer 2015; 14:65.
de Bie P, Ciechanover A . Ubiquitination of E3 ligases: self-regulation of the ubiquitin system via proteolytic and non-proteolytic mechanisms. Cell Death Differ 2011; 18:1393–1402.
Xue Y, Ren J, Gao X, ** C, Wen L, Yao X . GPS 2.0, a tool to predict kinase-specific phosphorylation sites in hierarchy. Mol Cell Proteomics 2008; 7:1598–1608.
Campaner S, Doni M, Hydbring P, et al. Cdk2 suppresses cellular senescence induced by the c-myc oncogene. Nat Cell Biol 2010; 12:54–59.
van Riggelen J, Felsher DW . Myc and a Cdk2 senescence switch. Nat Cell Biol 2010; 12:7–9.
Lents NH, Keenan SM, Bellone C, Baldassare JJ . Stimulation of the Raf/MEK/ERK cascade is necessary and sufficient for activation and Thr-160 phosphorylation of a nuclear-targeted CDK2. J Biol Chem 2002; 277:47469–47475.
Chambard JC, Lefloch R, Pouyssegur J, Lenormand P . ERK implication in cell cycle regulation. Biochim Biophys Acta 2007; 1773:1299–1310.
Gysin S, Lee SH, Dean NM, McMahon M . Pharmacologic inhibition of RAF→MEK→ERK signaling elicits pancreatic cancer cell cycle arrest through induced expression of p27Kip1. Cancer Res 2005; 65:4870–4880.
Meloche S, Pouyssegur J . The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene 2007; 26:3227–3239.
Yu Q, Geng Y, Sicinski P . Specific protection against breast cancers by cyclin D1 ablation. Nature 2001; 411:1017–1021.
Dulic V, Drullinger LF, Lees E, Reed SI, Stein GH . Altered regulation of G1 cyclins in senescent human diploid fibroblasts: accumulation of inactive cyclin E-Cdk2 and cyclin D1-Cdk2 complexes. Proc Natl Acad Sci USA 1993; 90:11034–11038.
Stein GH, Drullinger LF, Soulard A, Dulic V . Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol Cell Biol 1999; 19:2109–2117.
McConnell BB, Starborg M, Brookes S, Peters G . Inhibitors of cyclin-dependent kinases induce features of replicative senescence in early passage human diploid fibroblasts. Curr Biol 1998; 8:351–354.
Kong Y, Cui H, Ramkumar C, Zhang H . Regulation of senescence in cancer and aging. J Aging Res 2011; 2011:963172.
Cao J, Wang Y, Dong R, et al. Hypoxia-induced WSB1 promotes the metastatic potential of osteosarcoma cells. Cancer Res 2015; 75:4839–4851.
Kim HS, Kim MA, Hodgson D, et al. Concordance of ATM (ataxia telangiectasia mutated) immunohistochemistry between biopsy or metastatic tumor samples and primary tumors in gastric cancer patients. Pathobiology 2013; 80:127–137.
Angele S, Falconer A, Edwards SM, et al. ATM polymorphisms as risk factors for prostate cancer development. Br J Cancer 2004; 91:783–787.
Kim JH, Kim H, Lee KY, et al. Genetic polymorphisms of ataxia telangiectasia mutated affect lung cancer risk. Hum Mol Genet 2006; 15:1181–1186.
Stredrick DL, Garcia-Closas M, Pineda MA, et al. The ATM missense mutation p.Ser49Cys (c.146C>G) and the risk of breast cancer. Hum Mutat 2006; 27:538–544.
Hall J . The Ataxia-telangiectasia mutated gene and breast cancer: gene expression profiles and sequence variants. Cancer Lett 2005; 227:105–114.
Angele S, Treilleux I, Bremond A, Taniere P, Hall J . Altered expression of DNA double-strand break detection and repair proteins in breast carcinomas. Histopathology 2003; 43:347–353.
Grabsch H, Dattani M, Barker L, et al. Expression of DNA double-strand break repair proteins ATM and BRCA1 predicts survival in colorectal cancer. Clin Cancer Res 2006; 12:1494–1500.
Kang B, Guo RF, Tan XH, Zhao M, Tang ZB, Lu YY . Expression status of ataxia-telangiectasia-mutated gene correlated with prognosis in advanced gastric cancer. Mutat Res 2008; 638:17–25.
Tribius S, Pidel A, Casper D . ATM protein expression correlates with radioresistance in primary glioblastoma cells in culture. Int J Radiat Oncol Biol Phys 2001; 50:511–523.
Hsia SM, Yu CC, Shih YH, et al. Isoliquiritigenin as a cause of DNA damage and inhibitor of ataxia-telangiectasia mutated expression leading to G2/M phase arrest and apoptosis in oral squamous cell carcinoma. Head Neck 2016; 38 Suppl 1:E360–E371.
Flanagan JM, Munoz-Alegre M, Henderson S, et al. Gene-body hypermethylation of ATM in peripheral blood DNA of bilateral breast cancer patients. Hum Mol Genet 2009; 18:1332–1342.
Vo QN, Kim WJ, Cvitanovic L, Boudreau DA, Ginzinger DG, Brown KD . The ATM gene is a target for epigenetic silencing in locally advanced breast cancer. Oncogene 2004; 23:9432–9437.
Kairouz R, Clarke RA, Marr PJ, et al. ATM protein synthesis patterns in sporadic breast cancer. Mol Pathol 1999; 52:252–256.
Fang Z, Kozlov S, McKay MJ, et al. Low levels of ATM in breast cancer patients with clinical radiosensitivity. Genome Integr 2010; 1:9.
Dairkee SH, Ji Y, Ben Y, Moore DH, Meng Z, Jeffrey SS . A molecular 'signature' of primary breast cancer cultures; patterns resembling tumor tissue. BMC Genomics 2004; 5:47.
Lee SB, Kim JJ, Nam HJ, et al. Parkin regulates mitosis and genomic stability through Cdc20/Cdh1. Mol Cell 2015; 60:21–34.
Lee SB, Kim JJ, Chung JS, et al. Romo1 is a negative-feedback regulator of Myc. J Cell Sci 2011; 124:1911–1924.
Acknowledgements
We thank all members of Dr Lou's laboratory (Dr K Luo, Dr J Yuan, Dr H Pei, Dr B Qin, Dr T Liu, Dr H Zhang, Dr M Deng, Dr Y Li, Dr F Yuan, Dr W Guo, Dr L Zhang, Dr B Xu, Dr H Shi, S Nowsheen, and Y-H Lin) for their critical discussions. We thank Dr MS Kim and Dr CH Park for performing the animal experiment. We thank Dr CY Choi for WSB1 plasmids and Dr Y Ikeda for luciferase lentivirus plasmid. We thank Dr Kah Whye Peng and Diana L Escobar for bioluminescence-based IVIS system. We also thank Dr Z Zhang and JS Lee for ER-RAS-IMR-90 cells, and Dr Titia DeLange (The Rockefeller University) and Dr Hui-Kuan Lin (Wake Forest School of Medicine) for GST-ATM plasmids. This work was supported by 2015DFA30610, NIH grants CA130996, CA203561, and CA189666 to ZL.
Author information
Authors and Affiliations
Corresponding author
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
Tumorigenesis is induced by H-RasV12 through activation of the WSB1 in pre-malignant lesion. (PDF 421 kb)
Supplementary information, Figure S2
WSB1 regulates ATM after oncogenic stress. (PDF 270 kb)
Supplementary information, Figure S3
WSB1 regulates ATM by CDK2 under H-RasV12. (PDF 867 kb)
Supplementary information, Figure S4
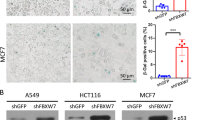
WSB1 negatively regulates ATM in various cancer cell lines. (PDF 322 kb)
Rights and permissions
About this article
Cite this article
Kim, J., Lee, S., Yi, SY. et al. WSB1 overcomes oncogene-induced senescence by targeting ATM for degradation. Cell Res 27, 274–293 (2017). https://doi.org/10.1038/cr.2016.148
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cr.2016.148
- Springer Nature Singapore Pte Ltd.
Keywords
This article is cited by
-
WSB1, as an E3 ligase, restrains myocardial ischemia–reperfusion injury by activating β-catenin signaling via promoting GSK3β ubiquitination
Molecular Medicine (2024)
-
HNRNPL Increases WSB1 mRNA Stability to Promote Proliferation and Lipid Droplets in Clear Cell Renal Cell Carcinoma
Cell Biochemistry and Biophysics (2024)
-
Identifying common transcriptome signatures of cancer by interpreting deep learning models
Genome Biology (2022)
-
Multilayered control of splicing regulatory networks by DAP3 leads to widespread alternative splicing changes in cancer
Nature Communications (2022)
-
Oncogene-induced senescence: a double edged sword in cancer
Acta Pharmacologica Sinica (2018)