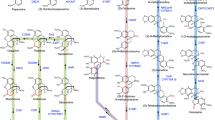
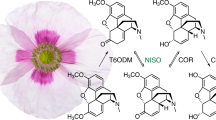
This opium poppy mutant provides chemical precursors for non-addictive analgesics.
Abstract
The opium poppy is a source of the pharmaceuticals codeine, morphine and their derived analgesics. Here we describe the initial characterization of the poppy mutant known as top1 (for ‘thebaine oripavine poppy 1’), which accumulates the morphine and codeine precursors thebaine and oripavine and does not complete their biosynthesis into morphine and codeine. The original discovery of top1 stimulated a re-engineering of the opioid industry in the island state of Tasmania, which grows over 40% of the world's licit opiates, in order to produce thebaine and oripavine efficiently from morphine-free poppy crops to provide precursors for highly effective analgesics and for treatment of opioid addiction.


Similar content being viewed by others
References
Parker, H. I., Blaschke, G. & Rapoport, H. Am. Chem. Soc. 94, 1276–1282 (1972).
Brochmann-Hanssen, E. Planta Medica 50, 343–345 (1984).
Kutchan, T. M. Gene 179, 73–81 (1996).
Zenk, M. H. in Organic Reactivity: Physical and Biological Aspects (eds Maskill, H., Golding, B. T. & Griffin, R. J.) 89–109 (Royal Society of Chemistry, Cambridge, UK, 1995).
Kutchan, T. M., Frick, S. & Weid, M. in Advances in Plant Biochemistry and Molecular Biology (eds Bohnert, H. J. & Nguyen, H. T.) (Elsevier Science, Oxford, in the press).
Bird, D. A., Franceschi, V. R. & Facchini, P. J. Plant Cell 15, 2626–2635 (2003).
De-Luca, V. & St Pierre, B. Trends Plant Sci. 5, 168–173 (2000).
Bock, A., Wanner, G. & Zenk, M. H. Planta 216, 57–63 (2002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Millgate, A., Pogson, B., Wilson, I. et al. Morphine-pathway block in top1 poppies. Nature 431, 413–414 (2004). https://doi.org/10.1038/431413a
Published:
Issue Date:
DOI: https://doi.org/10.1038/431413a
- Springer Nature Limited
This article is cited by
-
Insights into opium poppy (Papaver spp.) genetic diversity from genoty**-by-sequencing analysis
Scientific Reports (2022)
-
Chromatographic and spectroscopic study of impurities present in thebaine obtained from Papaver bracteatum Lindl.
Chemical Papers (2021)
-
Neopinone isomerase is involved in codeine and morphine biosynthesis in opium poppy
Nature Chemical Biology (2019)
-
Development of a Method to Extract Opium Poppy (Papaver somniferum L.) DNA from Heroin
Scientific Reports (2018)
-
Comparative analysis of Papaver somniferum genotypes having contrasting latex and alkaloid profiles
Protoplasma (2014)