Abstract
Purpose: Comparison of the influence of two different brain tumors (C6 and CNS1 glioma) on methotrexate (MTX) disposition in plasma, brain, and tumor tissue extracellular fluid (ECF).
Methods: Serial collection of plasma samples and brain ECF dialysates after i.v. bolus administration of MTX (50 mg kg-1) for 4 h. Quantitation of MTX concentrations by HPLC-UV.
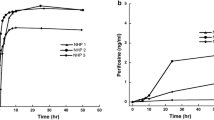
Results: Histological studies revealed a 3-fold higher number of blood vessels in CNS1 than in C6 tumor tissue. In vivo recoveries (reverse dialysis) were significantly different in tumor tissue (C6: 8.0 ± 3.8%; CNS1: 4.9 ± 2.5%), and in the contralateral hemisphere (C6: 6.0 ± 4.0%; CNS1: 3.9 ± 2.5%) between the two tumors. Area under the concentration–time curve (AUC) in plasma was 30% higher in CNS1 than in C6 due to a lower systemic clearance. Maximum MTX levels in brain tumor ECF were significantly higher in CNS1 than in C6, and decreased faster in CNS1 than in C6 tumor-bearing rats. Penetration in tumor ECF (AUCECF/AUCPlasma ratio) was similar in CNS1 and C6. MTX concentrations in contralateral hemisphere were significantly lower than in tumor tissue and dependent on tumor model.
Conclusion: C6 and CNS1 brain tumors have a distinct yet highly variable impact on MTX penetration in brain and brain tumor ECF.
Similar content being viewed by others
References
Shapiro WR, Voorhies RM, Hiesiger EM, Sher PB, Basler GA, Lipschutz LE: Pharmacokinetics of tumor cell exposure to [14C]methotrexate after intracarotid administration without and with hyperosmotic opening of the blood-brain and blood-tumor barriers in rat brain tumors: a quantitative autoradiographic study. Cancer Res 3: 694–701, 1988
Neuwelt EA, Rapoport SI: Modification of the blood–brain barrier in the chemotherapy of malignant brain tumors. Fed Proc 2: 214–219, 1984
Dukic S, Heurtaux T, Kaltenbach ML, Hoizey G, Lallemand A, Gourdier B, Vistelle R: Pharmacokinetics of methotrexate in the extracellular fluid of brain C6-glioma after intravenous infusion in rats. Pharm Res 8: 1219–1225, 1999
Jain RK: Transport of molecules across tumor vasculature. Cancer Metastasis Rev 4: 559–593, 1987
Groothuis DR, Fischer JM, Lapin G, Bigner DD, Vick NA: Permeability of different experimental brain tumor models to horseradish peroxidase. J Neuropathol Exp Neurol 2: 164–185, 1982
Devineni D, Klein-Szanto A, Gallo JM: In vivo microdialysis to characterize drug transport in brain tumors: analysis of methotrexate uptake in rat glioma-2 (RG-2)-bearing rats. Cancer Chemother Pharmacol 6: 499–507, 1996
de Lange EC, de Vries JD, Zurcher C, Danhof M, de Boer AG, Breimer DD: The use of intracerebral microdialysis for the determination of pharmacokinetic profiles of anticancer drugs in tumor-bearing rat brain. Pharm Res 12: 1924–1931, 1995
Neuwelt EA, Barnett PA, Frenkel EP: Chemotherapeutic agent permeability to normal brain and delivery to avian sarcoma virus-induced brain tumors in the rodent: observations on problems of drug delivery. Neurosurgery 14: 154–160, 1984
Benda P, Lightbody J, Sato G, Levine L, Sweet W: Differentiated rat glial cell strain in tissue culture. Science 161: 370–371, 1968
Kruse CA, Molleston MC, Parks EP, Schiltz PM, Kleinschmidt-DeMasters BK, Hickey WK: A rat glioma model, CNS-1, with invasive characteristics similar to those of human gliomas: a comparison to 9L gliosarcoma. J Neuro-Oncol 3: 191–200, 1994
Urien S: MicroPharm-K, a microcomputer interactive program for the analysis and simulation of pharmacokinetic processes. Pharm Res 12: 1225, 1995
Gibaldi M, Perrier D: Pharmacokinetics. In: Swarbrick J (ed) Drugs and Pharmaceutical Sciences. Marcel Decker, New York, pp 45–111, 1982
Motulsky H: Analyzing Data with GraphPad Prism. In: GraphPad Software, San Diego, USA, 1999
Morreale VM, Herman BH, Der-Minassian V, Palkovits M, Klubes P, Perry D, Csiffary A, Lee AP:Abrain–tumor model utilizing stereotactic implantation of a permanent cannula. J Neurosurg 6: 959–965, 1993
Kobayashi N, Allen N, Clendenon NR, Ko LW: An improved rat brain–tumor model. J Neurosurg 6: 808–815, 1980
Nugent LJ, Jain RK: Plasma pharmacokinetics and interstitial diffusion of macromolecules in a capillary bed. Am J Physiol 246: H129–H137, 1984
Hammarlund-Udenaes M, Paalzow LK, de Lange EC: Drug equilibration across the blood–brain barrier – pharmacokinetic considerations based on the microdialysis method. Pharm Res 2: 128–134, 1997
Bacic M, Edwards NA, Merrill MJ: Differential expression of vascular endothelial growth factor (Vascular Permeability Factor) forms in rat tissues. Growth Factors 12: 11–15, 1995
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dukic, S.F., Kaltenbach, M.L., Heurtaux, T. et al. Influence of C6 and CNS1 Brain Tumors on Methotrexate Pharmacokinetics in Plasma and Brain Tissue. J Neurooncol 67, 131–138 (2004). https://doi.org/10.1023/B:NEON.0000021820.12444.4c
Issue Date:
DOI: https://doi.org/10.1023/B:NEON.0000021820.12444.4c