Abstract
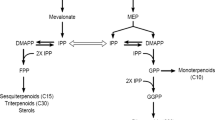
The effects of feeding of biosynthetic precursors and pathway specific inhibitors on anthraquinone (AQ) accumulation in fungal elicited cell cultures of Cinchona`Robusta' were studied. Addition of glyceraldehyde (1 mM), the initial precursor in the methyl-d-erythritol 4-phosphate (MEP) pathway, did not increase AQ accumulation, suggesting that the endogenous level of this precursor is not a limiting factor of AQ flux. It is proposed that AQs in Cinchona might be derived from the phenylpropanoid pathway, e.g. from caffeic acid. Addition of ferulic acid (1 mM) did not stimulate AQ accumulation, while addition of caffeic acid increased AQ accumulation by 48% compared to the control. The stimulating effect of feeding caffeic acid on AQ accumulation might be due to activation of other pathways. Addition of tectoquinone (2-methyl-anthraquinone) did not change the AQ patterns nor the shifts between AQs in control and tectoquinone-treated cell cultures. Addition of lovastatin, a specific inhibitor of the mevalonic acid pathway, did not inhibit the AQ accumulation. Clomazone, an inhibitor in the MEP pathway, inhibited the AQ accumulation, however. The simultaneous addition of lovastatin and clomazone inhibited both cell growth and AQ accumulation. These results further support the finding that isopentenyl diphosphate, which constitutes ring C of AQs in Cinchona `Robusta', is derived from the MEP pathway, and not from the mevalonic acid pathway.
Similar content being viewed by others
References
Alberts AW, Chen J, Kuron G, Huff V, Hoffman C, Rothrock J, Lopez M, Joshua H, Harris E, Petchad A, Monagan R, Currie S, Stapley E, Albers-Schönberg G, Hensens O, Hirschfield J, Hoogsteen K, Liesch J, Springer J (1980) Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc. Natl. Acad. Sci. USA 77: 3957-3961.
Bauch HJ, Leistner E (1978) Aromatic metabolites in cell suspension cultures of Galium mollugo. Planta Med. 33: 105-123.
Bochar DA, Friesen JA, Stauffacher CV, Rodwell VW (1999) Biosynthesis of mevalonic acid from acetyl-CoA. In: Barton D, Nakanishi K, eds. Comprehensive Natural Products Chemistry, Vol. 2. Oxford: Pergamon, pp. 15-44.
Croteau R (1992) Clomazone does not inhibit the conversion of isopentenyl pyrophosphate to geranyl, farnesyl, or geranylgeranyl pyrophosphate in vitro. Plant Physiol. 98: 1515-1517.
Duke SO, KenyonWH (1988) Effects of dimethazone (FMC 57020) on chloroplast development: II. Pigment synthesis and photosynthetic function in cow pea (Vigna unguiculta L.) primary leaves. Pestic. Biochem. Physiol. 25: 11-18.
Eichinger D, Bacher A, Zenk MH, Eisenreich W (1999) Quantitative assesment of metabolic flux by 13C NMR analysis. Biosynthesis of anthraquinones in Rubia tinctorum. J. Am. Chem. Soc. 121: 7469-7475.
Eisenreich W, Rohdich F, Bacher A (2001) Deoxyxylulose phosphate pathway to terpenoids. Trends Plant Sci. 6: 78-84.
Han Y, van der Heijden R, Lefeber AWM, Erkelens C, Verpoorte R (2002) Biosynthesis of anthraquinones in cell cultures of Cinchona 'Robusta' proceeds via the methylerythritol 4-phosphate pathway. Phytochemistry 59: 45-55.
Han Y, van der Heijden R, Verpoorte R (2001) Biosynthesis of anthraquinones in cell cultures of the Rubiaceae. Plant Cell Tiss. Org. Cult. 67: 201-220.
Leistner E (1985) Biosynthesis of chorismate-derived quinones in plant cell cultures. In: Neumann KH, Barz W, Reinhard E, eds. Primary and Secondary Metabolism of Plant Cell Cultures. Berlin/New York: Springer-Verlag, pp. 215-224.
Mueller C, Schwender J, Zeidler J, Lichtenthaler HK (2000) Properties and inhibition of the first two enzymes of the non-mevalonate pathway of isoprenoid biosynthesis. Biochem. Soc. Trans. 28: 792-793.
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 15: 473-497.
Ramos-Valdivia A, van der Heijden R, Verpoorte R (1997) Elicitormediated induction of anthraquinone biosynthesis and regulation of isopentenyl diphosphate isomerase and farnesyl diphosphate synthase activities in cell suspension cultures of Cinchona robusta. Planta 203: 155-161.
Rodriguez MC, Gruissem W (1999) Arachidonic acid alters tomato HMG expression and fruit growth and induces 3-hydroxy-3-methylglutaryl coenzyme A reductase-independent lycopene accumulation. Plant Physiol. 119: 41-48.
Rohmer M(1999) The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat. Prod. Rep. 16: 565-574.
Sandmann G, Böger P (1986) Interference of dimethazone with formation of terpenoid compounds. Z. Naturforsch. 41C: 729-732.
Schripsema J, Ramos-Valdivia AC, Verpoorte R (1999) Robustaquinones, novel anthraquinones from an elicited Cinchona robusta suspension culture. Phytochemistry 51: 55-60.
Schulte U, El Shagi H, Zenk MH (1984) Optimization of 19 Rubiaceae species in cell suspension cultures of Cinchona ledgeriana. Plant Cell Rep. 3: 51-54.
Scott JE, Weston LA, Chappell J, Hanley K (1994) Effects of clomazone on IPP isomerase and prenyl transferase activities in cell suspension cultures and cotyledons of Solanaceous species. Weed Sci. 42: 509-516.
Snoeijer W (2000) Flora of Pharmacognosy, December 2000. Leiden University, pp. 27.
Stalman M, Swolfs AEM, Klunder AJH, Croes AF, van der Plas LHW, Hagendoorn MJM, Wullems GJ (2001) Pyruvate stimulates anthraquinone biosynthesis in cell cultures of Morinda citrifolia. Ph.D Thesis. Univ. of Nijmegen, the Netherlands, pp. 53-72.
Van den Berg AJJ, Labadie RP (1989) Quinones. in: Harborne JB, ed. Methods in Plant Biochemistry, Vol 1. London: Academic Press Limited, pp. 451-491.
Wijnsma R, Verpoorte R (1986) Anthraquinones in the Rubiaceae. In: Herz W, Grisebach GW, Kirby Ch. Tamm, eds. Prog. Chem. Org. Nat. Prod., Vol. 49. New York/Vienna: Springer-Verlag, pp. 79-149.
Wijnsma R, Go JTKA, van Weerden IN, Harkes PAA, Verpoorte R, Baerheim-Svendsen A (1985) Anthraquinones as phytoalexins in cell and tissue cultures of Cinchona sp. Plant Cell Rep. 4: 241-244.
Zeidler J, Schwender J, Mueller C, Lichtenthaler HK (2000) The non-mevalonate isoprenoid biosynthesis of plants as a test system for drugs against malaria and pathogenic bacteria. Biochem. Soc. Trans. 28: 796-798.
Zenk MH, El-Shagi H, Schulte U (1975) Anthraquinone production by cell suspension cultures of Morinda citrifolia. Planta Med. (Suppl.): 79-101.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Han, YS., van der Heijden, R. & Verpoorte, R. Improved anthraquinone accumulation in cell cultures of Cinchona `Robusta' by feeding of biosynthetic precursors and inhibitors. Biotechnology Letters 24, 705–710 (2002). https://doi.org/10.1023/A:1015286117297
Issue Date:
DOI: https://doi.org/10.1023/A:1015286117297