Abstract
Background
The inverse relationship between GnRH transcript level and GABA neurons activity has suggested that GABA at the hypothalamic level may exert a suppressive effect on subsequent steps of the GnRH biosynthesis. In the present study, we analyzed the effects of GABA type A receptor agonist (muscimol) or antagonist (bicuculline) on molecular mechanisms governing GnRH/LH secretion in follicular-phase sheep.
Methods
ELISA technique was used to investigate the effects of muscimol and/or bicuculline on levels of post-translational products of genes encoding GnRH ligand and GnRH receptor (GnRHR) in the preoptic area (POA), anterior (AH) and ventromedial (VMH) hypothalamus, stalk/median eminence (SME), and GnRHR in the anterior pituitary (AP). Real-time PCR was chosen for determination of the effect of drugs on kisspeptin (Kiss 1) mRNA level in POA and VMH including arcuate nucleus (VMH/ARC), and on Kiss1 receptor (Kiss1r) mRNA abundance in POA-hypothalamic structures. These analyses were supplemented by RIA method for measurement of plasma LH concentration.
Results
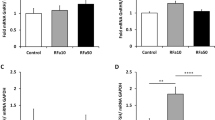
The study demonstrated that muscimol and bicuculline significantly decreased or increased GnRH biosynthesis in all analyzed structures, respectively, and led to analogous changes in plasma LH concentration. Similar muscimol- and bicuculline-related alterations were observed in levels of GnRHR. However, the expression of Kiss 1 and Kiss1r mRNAs in selected POA-hypothalamic areas of either muscimol- and bicuculline-treated animals remained unaltered.
Conclusions
Our data suggest that GABAergic neurotransmission is involved in the regulatory pathways of GnRH/GnRHR biosynthesis and then GnRH/LH release in follicular-phase sheep conceivably via indirect mechanisms that exclude involvement of Kiss 1 neurons.
Similar content being viewed by others
References
Sieghart W. Structure and pharmacology of gamma-aminobutyric acid A receptor subtypes. Pharmacol Rev 1995;47(2):181–234.
Mott DD, Lewis DV. The pharmacology and function of central GABAB receptors. Int Rev Neurobiol 1994;36:97–223.
Pape JR, Skynner MJ, Herbison AE. Profiling gamma-aminobutyric acid (GABA (A)) receptor subunit mRNA expression in postnatal gonadotropin-releasing hormone (GnRH) neurons of the male mouse with single cell RT-PCR. Neuroendocrinology 2001;74(5):300–8.
Moragues N, Ciofi P, Lafon P, Tramu G, Garret M. GABA-A receptor epsilon subunit expression in identified peptidergic neurons of the rat hypothalamus. Brain Res 2003;967(1–2):285–9, doi: https://doi.org/10.1016/S0006-8993(02)04270-1.
Sliwowska JH, Billings HJ, Goodman RL, Lehman MN. Immunocytochemical colocalization of GABA-B receptor subunits in gonadotropin-releasing hormone neurons of the sheep. Neuroscience 2006;141(1):311–9, doi: https://doi.org/10.1016/j.neuroscience.2006.03.039.
Zhang C, Bosch MA, Rønnekleiv OK, Kelly MJ. Gamma-aminobutyric acid B receptor mediated inhibition of gonadotropin-releasing hormone neurons is suppressed by kisspeptin-G protein-coupled receptor 54 signaling. Endocrinology 2009;150(5):2388–94, doi: https://doi.org/10.1210/en.2008-1313.
Leranth C, MacLusky NJ, Sakamoto H, Shanabrough M, Naftolin F. Glutamic acid decarboxylase-containing axons synapse on LHRH neurons in the rat medial preoptic area. Neuroendocrinology 1988;40(6):536–9, doi: https://doi.org/10.1159/000124127.
Jansen HT, Cutter C, Hardy S, Lehman MN, Goodman RL. Seasonal plasticity within the gonadotropin-releasing hormone (GnRH) system of the ewe: changes in identified GnRH inputs and glial association. Endocrinology 2003;144(8):3663–76, doi: https://doi.org/10.1210/en.2002-0188.
Jackson GL, Wood SG, Kuehl DE. A gamma-aminobutyric acid agonist reverses the negative feedback effect of testosterone on gonadotropin-releasing hormone and luteinizing hormone secretion in the male sheep. Endocrinology 2000;141(11):3940–5, doi: https://doi.org/10.1210/endo.141.11.7754.
Jackson GL, Kuehl D. Effects of applying gamma-aminobutyric acid (B) drugs into the medial basal hypothalamus on basal luteinizing hormone concentrations and on luteinizing hormone surges in the female sheep. Biol Reprod 2004;70(2):334–9, doi: https://doi.org/10.1095/biolreprod.103.021311.
Tomaszewska-Zaremba D, Przekop F. Effects of GABAB receptor modulation on gonadotropin-releasing hormone and beta-endorphin release, and on catecholaminergic activity in the ventromedial hypothalamus-infundibular nucleus region of anestrous ewes. J Neuroendocrinol 2005;17(1):49–56, doi: https://doi.org/10.1111/j.1365-2826.2005.01276.x.
Moenter SM, De Fazio RA. Endogenous gamma-aminobutyric acid can excite gonadotropin-releasing hormone neurons. Endocrinology 2005;146(12):5374–9, doi: https://doi.org/10.1210/en.2005-0788.
Scott CJ, Clarke IJ. Inhibition of luteinizing hormone secretion in ovariectomized ewes during the breeding season by gamma-aminobutyric acid (GABA) is mediated by GABA-A receptors, but not GABA-B receptors. Endocrinology 1993;132(4):1789–96, doi: https://doi.org/10.1210/endo.132.4.8384997.
Tomaszewska-Zaremba D, Mateusiak K, Przekop F. The role of GABAA receptors in the neural systems of the medial preoptic area in the control of GnRH release in ewes during follicular phase. Anim Reprod Sci 2003;77(1–2):71–83.
Lin YS, Li XF, Shao B, Hu MH, Goundry AL, Jeyaram A, et al. The role of GABAergic signalling in stress-induced suppression of gonadotrophin-releasing hormone pulse generator frequency in female rats. J Neuroendocrinol 2012;24(3):477–88, doi: https://doi.org/10.1111/j.1365-2826.2011.02270.x.
De Fazio RA, Heger S, Ojeda SR, Moenter SM. Activation of A-type gamma-aminobutyric acid receptors excites gonadotropin-releasing hormone neurons. Mol Endocrinol 2002;16(12):2872–91, doi: https://doi.org/10.1210/me.2002-0163.
Bhattarai JP, Park SA, Park JB, Lee SY, Herbison AE, Ryu PD, et al. Tonic extrasynaptic GABA(A) receptor currents control gonadotropin-releasing hormone neuron excitability in the mouse. Endocrinology 2011;152(4):1551–61, doi: https://doi.org/10.1210/en.2010-1191.
Herbison AE, Moenter SM. Depolarising and hyperpolarizing actions of GABAA receptor activation on gonadotropin-releasing hormone neurons: towards an emerging consensus. J Neuroendocrinol 2011;23:557–69, doi: https://doi.org/10.1111/j.1365-2826.2011.02145.x.
Watanabe M, Sakuma Y, Kato M. GABAA receptors mediate excitation in adult rat GnRH. neurons. Biol Reprod. 2009;81(2):327–32, doi: https://doi.org/10.1095/biolreprod.108.074583.
Nakane R, Oka Y. Excitatory action of GABA in the terminal nerve gonadotropin-releasing. Hormone neurons. J Neurophysiol 2010;103(3):1375–84, doi: https://doi.org/10.1152/jn.00910.2009.
Han SK, Todman MG, Herbison AE. Endogenous GABA release inhibits the firing of adult gonadotropin-releasing hormone neurons. Endocrinology 2004;145(2):495–9, doi: https://doi.org/10.1210/en.2003-1333.
Dobson H, Ghuman S, Prabhakar S, Smith R. A conceptual model of the influence of stress on female reproduction. Reproduction 2003;125(2):151–63.
Sullivan SD, Moenter SM. Gamma-aminobutyric acid neurons integrate and rapidly transmit permissive and inhibitory metabolic cues to gonadotropin-releasing hormone neurons. Endocrinology 2004;145(3):1194–202, doi: https://doi.org/10.1210/en.2003-1374.
Sullivan SD, Moenter SM. GABAergic integration of progesterone and androgen feedback to gonadotropin-releasing hormone neurons. Biol Reprod 2005;72(1):33–41, doi: https://doi.org/10.1095/biolreprod.104.033126.
Ciechanowska M, Łapot M, Malewski T, Mateusiak K, Misztal T, Przekop F. Effects of GABAA receptor modulation on the expression of GnRH gene and GnRH receptor (GnRH-R) gene in the hypothalamus and GnRH-R gene in the anterior pituitary gland of follicular-phase ewes. Anim Reprod Sci 2009;111:235–48, doi: https://doi.org/10.1016/j.anireprosci.2008.03.006.
Ferreira SA, Hileman SM, Kuehl DE, Jackson GL. Effects of dialyzing y-aminobutyric acid receptor antagonists into the medial preoptic and arcuate ventromedial region on luteinizing hormone release in male sheep. Biol Reprod 1998;58(4):1038–46.
Caraty A, Smith JT, Lomet D, Ben Said S, Morrissey A, Cognie J, et al. Kisspeptin synchronizes preovulatory surges in cyclical ewes and causes ovulation in seasonally acyclic ewes. Endocrinology 2007;148:5258–67, doi: https://doi.org/10.1210/en.2007-0554.
Pielecka-Fortuna J, Moenter SM. Kisspeptin increases gamma-aminobutyric acidergic and glutamatergictransmission directly to gonadotropin-releasing hormone neurons in an estradiol-dependent manner. Endocrinology 2010;151:291–300, doi: https://doi.org/10.1210/en.2009-0692.
Kurian JR, Keen KL, Guerriero KA, Terasawa E. Tonic control of kisspeptin release in prepubertal monkeys: implication to the mechanism of puberty onset. Endocrinology 2012;153:3331–6, doi: https://doi.org/10.1210/en.2012-1221.
Leonhardt S, Seong JY, Kim K, Thorun Y, Wuttke W, Jarry H. Activation of central GABAA but not GABAB-receptors rapidly reduces pituitary LH release and GnRH gene expression in the preoptic/anterior hypothalamic area of ovariectomized rats. Neuroendocrinology 1995;61(6):655–62, doi: https://doi.org/10.1159/000126892.
Kang SH, Seong JY, Cho S, Cho H, Kim K. Acute increases of GABAergic neurotransmission exerts a stimulatory effect on GnRH gene expression in the preoptic/anterior hypothalamic area of ovariectomized, estrogen- and progesterone-treated adult female rats. Neuroendocrinology 1995;61:486–92, doi: https://doi.org/10.1159/000126871.
Fueshko SM, Key S, Wray S. Luteinizing hormone releasing hormone (LHRH) neurons maintained in nasal explants decrease LHRH messenger ribonucleic acid levels after activation of GABAA receptors. Endocrinology 1998;139(6):2734–40, doi: https://doi.org/10.1210/endo.139.6.6034.
Seong JY, Kang SS, Kam K, Han Y-G, Kwon HB, Ryn K, et al. Differential regulation of gonadotropin-releasing hormone (GnRH) receptor expression in the posterior mediobasal hypothalamus by steroid hormones: implication of GnRH neuronal activity. Mol Brain Res 1998;53:226–35, doi: https://doi.org/10.1016/S0169-328X(97)00297-0.
Roth C, Jung K, Kim K, Arias P, Moguilevsky J, Jarry H, et al. Involvement of gamma amino butyric acid (GABA) in the postnatal function of the GnRH pulse generator as determined on the basis of GnRH and GnRH-receptor gene expression in the hypothalamus and pituitary. Exp Clin Endocrinol Diab 1997;105(6):353–8, doi: https://doi.org/10.1055/s-0029-1211778.
Hawken PAR, Jorre de St. Jorre T, Rodger J, Esmaili T, Blache D, et al. Rapid induction of cell proliferation in the adult female ungulate brain (Ovis aries) associated with activation of the reproductive axis by exposure to unfamiliar males. Biol Reprod 2009;80:1146–51, doi: https://doi.org/10.1095/biolreprod.108.075341.
Traczyk W, Przekop F. Methods of investigation of the function of the hypothalamus and hypophysis in chronic experiments in sheep. Acta Physiol Pol 1963;14:227–36.
Welento J, Steyn S, Milart Z. Observation on the stereotaxic configuration of the hypothalamus nuclei in sheep. Anat Anz 1969;124(1):1–27.
Koressaar T, Remm M. Enhancements and modifications of primer design program Primer3. Bioinformatics 2007;23(10):1289–91, doi: https://doi.org/10.1093/bioinformatics/btm091.
Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, et al. Primer3 — new capabilities and interfaces. Nucleic Acid Res 2012;40(15):e115, doi: https://doi.org/10.1093/nar/gks596.
Peletto S, Bertuzzi S, Campanella C, Modesto P, Maniaci M, Bellino C, et al. Evaluation of internal genes for quantitative expression analysis by Real-Time PCR in ovine whole blood. Int J Mol Sci 2011;12:7732–47, doi: https://doi.org/10.3390/ijms12117732.
Livak KJ, Smittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and 2-Δ Δ C T method. Methods 2001;25:402–8, doi: https://doi.org/10.1006/meth.2001.1262.
Zhao S, Fernald RD. Comprehensive algorithm for quantitative real-time polymerase chain reaction. J Comput Biol 2005;12:1047–64, doi: https://doi.org/10.1089/cmb.2005.12.1047.
Stupnicki R, Madej A. Radioimmunoassay of LH in blood plasma of farm animals. Endokrinologie 1976;68(1):6–13.
Viguie C, Caraty A, Locatelli A, Malpaux B. Regulation of luteinizing hormone-releasing hormone (LHRH) secretion by melatonin in the ewe. I. Simultaneous delayed increase in LHRH and luteinizing hormone pulsatile secretion. Biol Reprod 1995;52(5):1114–20.
Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev 1998;19(3):302–30, doi: https://doi.org/10.1210/edrv.19.3.0332.
Thanky NR, Slater R, Herbison AE. Sex differences in estrogen-dependent transcription of gonadotropin releasing hormone (GnRH) gene revealed in GnRH transgenic mice. Endocrinology 2003;144(8):3351–8, doi: https://doi.org/10.1210/en.2001-211342.
Maier T, Güell M, Serrano L. Correlation of mRNA and protein incomplex biological samples. FEBS Lett 2009;24:3966–73, doi: https://doi.org/10.1016/j.febslet.2009.10.036.
Caldani M, Batailler M, Thiéry JC, Dubois MP. LHRH-immunoreactive structures in the sheep brain. Histochemistry 1988;89(2):129–39.
Ciechanowska M, Łapot M, Mateusiak K, Przekop F. Neuroendoendocrine regulation of GnRH release and expression of GnRH and GnRH receptor genes in the hypothalamus-pituitary unit in different physiological states. Reprod Biol 2010;10(2):85–124.
Herman AP, Tomaszewska-Zaremba D. Effect of endotoxin on the expression of GnRH and GnRHR genes in the hypothalamus and anterior pituitary gland of anestrous ewes. Anim Reprod Sci 2010;120(1–4):105–11, doi: https://doi.org/10.1016/j.anireprosci.2010.03.011.
Ciechanowska M, Łapot M, Mateusiak K, Paruszewska E, Malewski T, Przekop F. Biosynthesis of gonatropin-releasing hormone (GnRH) and GnRH receptor (GnRHR) in hypothalamic-pituitary unit of anestrous and cyclic ewes. Can J Physiol Pharmacol 2017;95(2):178–84, doi: https://doi.org/10.1139/cjpp-2016-0137.
Harris TG, Robinson JE, Evans NP, Skinner DC, Herbison AE. Gonadotropin-releasing hormone messenger ribonucleic acid expression changes before the onset of the estradiol-induced luteinizing hormone surge in the ewe. Endocrinology 1998;139(1):57–64, doi: https://doi.org/10.1210/endo.139.1.5662.
Robinson JE, Healey AE, Harris TG, Messent EA, Skinner DC, Taylor JA, et al. The negative feedback action of progesterone on luteinizing hormone release is not associated with changes in GnRH mRNA expression in the Ewe. J Neuroendocrinol 2000;12(2):121–9, doi: https://doi.org/10.1046/j.1365-2826.2000.00426.x.
Ferreira SA, Scott CJ, Kuehl DE, Jackson GL. Differential regulation of luteinizg hormone release by gamma-aminobutyric acid receptor subtypes in the arcuate-ventromedial region of castrated ram. Endocrinology 1996;137:3453–60, doi: https://doi.org/10.1210/endo.137.8.8754774.
Ghuman SPS, Jones DN, Prabhakar S, Smith RF, Dobson H. GABA control of GnRH release from the ewe hypothalamus in vitro: sensitivity of oestradiol. Reprod Domest Anim 2008;43:531–41, doi: https://doi.org/10.1111/j.1439-0531.2007.00948.x.lin.
Sim JA, Skynner MJ, Pape JR, Herbison AE. Late postnatal reorganization of GABAA receptor signaling in native GnRH neurons. Eur J Neurosci 2000;12:3497–504, doi: https://doi.org/10.1046/j.1460-9568.2000.00261.x.
Temple JL, Wray S. Developmental changes in GABA receptor subunit composition within the gonadotropin-releasing hormone-1 neuronal system. J Neuroendocrinol 2005;17:591–9, doi: https://doi.org/10.1111/j.1365-2826.2005.01348.x.
Horvath T, Naftolin F, Leranth C. Luteinizing homone-releasing hormone and gamma-aminobutyric acid neurons in the medial preoptic area are synaptic targets of dopamine axons originating in anterior periventricular areas. J Neurondocrinol 1993;5:71–9.
Kanasaki H, Tumurbaatar T, Oride A, Hara T, Okada H, Kyo S. Gamma-aminobutyric acidA receptor agonist, muscimol, increases Kiss-1 gene expression in hypothalamic cell models. Reprod Med Biol 2017;16:386–91, doi: https://doi.org/10.1002/rmb2.12061.
Constantin S, Iremonger KJ, Herbison AE. In vivo recordings of GnRH neuron firing reveal heterogeneity and dependence upon GABAA receptor signaling. J Neurosci 2013;33:9394–401, doi: https://doi.org/10.1523/JNEUROSCI.0533-13.2013.
Watanabe M, Fukuda A, Nabekura J. The role of GABA in the regulation of GnRH neurons. Front Neurosci 2014;8:387, doi: https://doi.org/10.3389/fnins.2014.00387.
De Paolo LV, King RA, Carrollo AJ. In vivo and in vitro examination of an autoregulatory mechanism for luteinizing hormone-releasing hormone. Endocrinology 1987;120:272–9, doi: https://doi.org/10.1210/endo-120-1-272.
Sarkar DK. In vivo secretion of LHRH in ovariectomized rats is regulated by a possible autofeedback mechanism. Neuroendocrinology 1987;45(6):510–3, doi: https://doi.org/10.1159/000124783.
Ciechanowska M, Łapot M, Antkowiak B, Mateusiak K, Paruszewska E, Malewski T, et al. Effect of short-term and prolonged stress on the biosynthesis of gonadotropin-releasing hormone (GnRH) and GnRH receptor (GnRHR) in the hypothalamus and GnRHR in the pituitary of ewes during various physiological states. Anim Reprod Sci 2016;174:65–72, doi: https://doi.org/10.1016/j.anireprosci.2016.09.006.
Turzillo AM, Nolan TE, Nett TM. Regulation of gonadotropin-releasing hormone (GnRH) receptor gene expression in sheep: interaction of GnRH and estradiol. Endocrinology 1998;139(12):4890–4, doi: https://doi.org/10.1210/endo.139.12.6344.
Cheng KW, Ngan ESW, Kang SK, Chow BKC, Leung PCK. Transcriptional down-regulation of human gonadotropin-releasing hormone (GnRH) receptor gene by GnRH: role of protein kinase C and activating protein 1. Endocrinology 2000;141:3611–22, doi: https://doi.org/10.1210/endo.141.10.7730.
Vizcarra JA, Wettemann RP, Braden TD, Turzillo AM, Nett TM. Effect of gonadotropin-releasing hormone (GnRH) pulse frequency on serum and pituitary concentrations of luteinizing hormone and follicle stimulating hormone, GnRH receptors, and messenger ribonucleic acid for gonadotropin subunits in cows. Endocrinology 1997;138(2):594–601, doi: https://doi.org/10.1210/endo.138.2.4938.
Kirkpatrick BL, Esquivel E, Moss GE, Hamernik DL, Wise ME. Estradiol and gonadotropin-releasing hormone (GnRH) interact to increase GnRH receptor expression in ovariectomized ewes after hypothalamic-pituitary disconnection. Endocrine 1998;8(3):225–9, doi: https://doi.org/10.1385/ENDO:8:3:225.
Kaiser UB, Jakubowiak A, Steinberger A, Chin WW. Differential effects of gonadotropin-releasing hormone (GnRH) pulse frequency on gonadotropin subunits and GnRH receptor messenger ribonucleic acid levels in vitro. Endocrinology 1997;138(3):1224–31, doi: https://doi.org/10.1210/endo.138.3.4968.
Gajewska A, Kochman K, Lerrant Y, Kochman H, Counis R. Modulation of luteinizing hormone subunit gene expression by intracerebroventricular microinjection of gonadotropin-releasing hormone or beta-endorphin in female rats. Biochim Biophys Acta 2000;1523(2–3):217–24, doi: https://doi.org/10.1016/S0304-4165(00)00125-2.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ciechanowska, M.O., Łapot, M., Kowalczyk, M. et al. Does kisspeptin participate in GABA-mediated modulation of GnRH and GnRH receptor biosynthesis in the hypothalamic-pituitary unit of follicular-phase ewes?. Pharmacol. Rep 71, 636–643 (2019). https://doi.org/10.1016/j.pharep.2019.02.019
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.pharep.2019.02.019