Abstract
Background
The human dopamine D2 receptor gene has three polymorphic variants that alter its amino acid sequence: alanine substitution by valine in position 96 (V96A), proline substitution by serine in position 310 (P310S) and serine substitution by cysteine in position 311 (S311C). Their functional role has never been the object of extensive studies, even though there is some evidence that their occurrence correlates with schizophrenia.
Methods
The HEK293 cell line was transfected with dopamine D1 and D2 receptors (or genetic variants of the D2 receptor), coupled to fluorescent proteins which allowed us to measure the extent of dimerization of these receptors, using a highly advanced biophysical approach (FLIM-FRET). Additionally, Fluoro-4 AM was used to examine changes in the level of calcium release after ligand stimulation of cells expressing different combinations of dopamine receptors.
Results
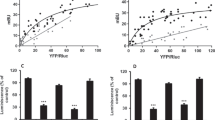
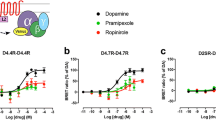
Using FLIM-FRET experiments we have shown that in HEK 293 expressing dopamine receptors, polymorphic mutations in the D2 receptor play a role in dimmer formation with the dopamine D1 receptor. The association level of dopamine receptors is affected by ligand administration, with variable effects depending on polymorphic variant of the D2 dopamine receptor. We have found that the level of heteromer formation is reflected by calcium ion release after ligand stimulation and have observed variations of this effect dependent on the polymorphic variant and the ligand.
Conclusion
The data presented in this paper support the hypothesis on the role of calcium signaling regulated by the D1–D2 heteromer which may be of relevance for schizophrenia etiology.
Similar content being viewed by others
References
Fuxe K, Marcellino D, Borroto-Escuela DO, Frankowska M, Ferraro L, Guidolin D, et al. The changing world of G protein-coupled receptors: from monomers to dimers and receptor mosaics with allosteric receptor-receptor interactions. J Recept Signal Transduct Res 2010;30(5):272–83.
Fuxe K, Borroto-Escuela DO, Romero-Fernandez W, Palkovits M, Tarakanov AO, Ciruela F, et al. Moonlighting proteins and protein-protein interactions as neurotherapeutic targets in the G protein-coupled receptor field. Neuropsychopharmacology 2014;39(1): 131–55.
Marsango S, Varela MJ, Milligan G. Approaches to characterize and quantify oligomerization of GPCRs. Methods Mol Biol 2015;1335:95–105.
Dreher JK, Jackson DM. Role of D1 and D2 dopamine receptors in mediating locomotor activity elicited from the nucleus accumbens of rats. Brain Res 1989;487(2):267–77.
Kita K, Shiratani T, Takenouchi K, Fukuzako H, Takigawa M. Effects of D1 and D2 dopamine receptor antagonists on cocaine-induced self-stimulation and locomotor activity in rats. Eur Neuropsychopharmacol 1999;9(1–2):1–7.
Aizman O, Brismar H, Uhlén P, Zettergren E, Levey AI, Forssberg H, et al. Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat Neurosci 2000;3(3):226–30.
Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Hervé D, Valjent E, et al. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci 2008;28(22):5671–85.
Deng YP, Lei WL, Reiner A. Differential perikaryal localization in rats of D1 and D2 dopamine receptors on striatal projection neuron types identified by retrograde labeling. J Chem Neuroanat 2006;32(2–4):101–16.
Hasbi A, Fan T, Alijaniaram M, Nguyen T, Perrault ML, O’Dowd BF, et al. Calcium signaling cascade links dopamine D1–D2 receptor heteromer to striatal BDNF production and neuronal growth. Proc Natl Acad Sci USA 2009; 106(50):21377–82.
Lee SP, So CH, Rashid AJ, Varghese G, Cheng R, Lanca AJ, et al. Dopamine D1 and D2 receptor co-activation generates a novel phospholipase C-mediated calcium signal. J Biol Chem 2004;279(34):35671–8.
Ng J, Rashid AJ, So CH, O’Dowd BF, George SR. Activation of calcium/calmodulin-dependent protein kinase IIalpha in the striatum by the heteromeric D1–D2 dopamine receptor complex. Neuroscience 2010;165(2):535–41.
Perrault ML, Hasbi A, Alijaniaram M, Fan T, Varghese G, Fletcher PJ, et al. The dopamine D1–D2 receptor heteromer localizes in dynorphin/enkephalin neurons: increased high affinity state following amphetamine and in schizophrenia. J Biol Chem 2010;285(47):36625–34.
Svenningsson P, Fredholm BB, Bloch B, Le Moine C. Co-stimulation of D(1)/D(5) and D(2) dopamine receptors leads to an increase in c-fos messenger RNA in cholinergic interneurons and a redistribution of c-fos messenger RNA in striatal projection neurons. Neuroscience 2000;98(4):749–57.
Dziedzicka-Wasylewska M, Faron-Górecka A, Andrecka J, Polit A, Kuśmider M, Wasylewski Z. Fluorescence studies reveal heterodimerization of dopamine D1 and D2 receptors in the plasma membrane. Biochemistry 2006;45(29):8751–9.
So CH, Varghese G, Curley KJ, Kong MM, Alijaniaram M, Ji X. D1 and D2 dopamine receptors form heterooligomers and cointernalize after selective activation of either receptor. Mol Pharmacol 2005;68(3):568–78.
George SR, O’Dowd BF. A novel dopamine receptor signaling unit in brain: heterooligomers of D1 and D2 dopamine receptors. Sci World J 2007;7:58–63.
Lidow MS. Calcium signaling dysfunction in schizophrenia: a unifying approach. Brain Res Rev 2003;43(1):70–84.
Seeman P. Dopamine receptors and the dopamine hypothesis of schizophrenia. Synapse 1987;1(2):133–52.
Issa G, Wilson C, Terry Jr. AV, Pillai A. An inverse relationship between cortisol and BDNF levels in schizophrenia: data from human postmortem and animal studies. Neurobiol Dis 2010;39(3):327–33.
**dal RD, Pillai AK, Mahadik SP, Eklund K, Montrose DM, Keshavan MS. Decreased BDNF in patients with antipsychotic naïve first episode schizophrenia. Schizophr Res 2010;119(1–3):47–51.
Novak G, Seeman P, Tallerico T. Schizophrenia: elevated mRNA for calcium-calmodulin-dependent protein kinase IIbeta in frontal cortex. Brain Res Mol Brain Res 2000;82(1–2):95–100.
Lee KW, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci USA 2006;103(9):3399–404.
Rashid AJ, So CH, Kong MM, Furtak T, El-Ghundi M, Cheng R, et al. D1–D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci USA 2007;104 (2):654–9.
Weickert CS, Hyde TM, Lipska BK, Herman MM, Weinberger DR, Kleinman JE. Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry 2003;8(6):592–610.
Sullinan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry 2003;60(12):1187–92.
Arinami T, Itokawa M, Enguchi H, Tagaya H, Yano S, Shimizu H, et al. Association of dopamine D2 receptor molecular variant with schizophrenia. Lancet 1994;343(8899):703–4.
Cravchik A, Sibley DR, Gejman PV. Functional analysis of the human D2 dopamine receptor missense variants. J Biol Chem 1996;271(42):26013–7.
Cravchik A, Sibley DR, Gejman PV. Analysis of neuroleptic binding affinities and potencies for the different human D2 dopamine receptor missense variants. Pharmacogenetics 1999;9(1):17–23.
Glatt SJ, Faraone SV, Tsuang MT. Meta-analysis identifies an association between the dopamine D2 receptor gene and schizophrenia. Mol Psychiatry 2003;8(11):911–5.
Faron-Górecka A, Górecki A, Kuśmider M, Wasylewski Z, Dziedzicka-Wasylewska M. The role of D1–D2 receptor hetero-dimerization in the mechanism of action of clozapine. Eur Neuropsychopharmacol 2008;18(9):682–91.
Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol 2002;20(1):87–90.
Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 2002;296 (5569):913–6.
Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. NY: Cold Spring Harbor Laboratory Press; 1989.
Koushik SV, Chen H, Thaler C, Paul HR, Vogel SS. Cerulean, venus, and venusY67C FRET reference standards. Biophys J 2006;91(12):99–101.
Sun Y, Day RN, Periasamy A. Investigating protein-protein interactions in living cells using fluorescence lifetime imaging microscopy. Nat Protoc 2011;6(9):1324–40.
Broussard JA, Rappaz B, Webb DJ, Brown CM. Fluorescence resonance energy transfer microscopy as demonstrated by measuring the activation of the serine/threonine kinase Akt. Nat Protoc 2013;8(2):265–81.
Tramier M, Zahid M, Mevel JC, Masse MJ. Coppey-Moisan M. Sensitivity of CFP/YFP and GFP/mCherry pairs to donor photobleaching on FRET determination by fluorescence lifetime imaging microscopy in living cells. Microsc Res Tech 2006;69(11):933–9.
Padilla-Parra S, Audugé N, Lalucque H, Mevel JC, Coppey-Moisan M, Tramier M. Quantitative comparison of different fluorescent protein couples for fast FRET-FLIM acquisition. BiophysJ 2009;97(8):2368–76.
Katritch V, Cherezov V, Stevens RC. Diversity and modularity of G protein-coupled receptor structures. Trends Pharmacol Sci 2012;33(1):17–27.
Shi L, Javitch JA. The second extracellular loop of the dopamine D2 receptor lines the binding-site crevice. Proc Natl Acad Sci USA 2004;101(2):440–5.
O’Dowd BF, Ji X, Nguyen T, George SR. Two amino acids in each of D1 and D2 dopamine receptor cytoplasmic regions are involved in D1–D2 heteromer formation. Biochem Biophys Res Commun 2012;417(1):23–8.
Granier S, Kim S, Shafer AM, Ratnala VR, Fung JJ, Zare RN, et al. Structure and conformational changes in the C-terminal domain of the beta2-adrenoceptor: insights from fluorescence resonance energy transfer studies. J Biol Chem 2007;282(18):13895–905.
Hoffmann C, Gaietta G, Bünemann M, Adams SR, Oberdorff-Maass S, Behr B, et al. A FlAsH-based FRET approach to determine G protein-coupled receptor activation in living cells. Nat Methods 2005;2(3):171–6.
Akrap N, Seidel T, Barisas BG. Förster distances for fluorescence resonant energy transfer between mCherry and other visible fluorescent proteins. Anal Biochem 2010;402(1):105–6.
Zürn A, Zabel U, Vilardaga JP, Schindelin H, Lohse MJ, Hoffmann C. Fluorescence resonance energy transfer analysis of alpha 2a-adrenergic receptor activation reveals distinct agonist-specific conformational changes. Mol Pharmacol 2009;75(3):534–41.
Itokawa M, Arinami T, Toru M. Advanced research on dopamine signaling to develop drugs for the treatment of mental disorders: ser311Cys polymorphisms of the dopamine D2-receptor gene and schizophrenia. J Pharmacol Sci 2010;114(1):6–16.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Błasiak, E., Łukasiewicz, S., Szafran-Pilch, K. et al. Genetic variants of dopamine D2 receptor impact heterodimerization with dopamine D1 receptor. Pharmacol. Rep 69, 235–241 (2017). https://doi.org/10.1016/j.pharep.2016.10.016
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.pharep.2016.10.016