Abstract
Depression is one of the most common mental disorders and social issue worldwide. Although there are many antidepressants available, the effectiveness of the therapy is still a serious issue. Moreover, there are many limitations of currently used antidepressants, including slow onset of action, numerous side effects, or the fact that many patients do not respond adequately to the treatment. Therefore, scientists are searching for new compounds with different mechanisms of action. Numerous data indicate the important role of glutamatergic, GABA-ergic, and cholinergic receptors in the pathomechanism of major depressive disorder. This review presents the role of glutamatergic, GABA-ergic, and cholinergic receptors in depression and antidepressant-like effect.
Similar content being viewed by others
References
WHO Depression. http://www.who.int/topics/depression/en/ (accessed May 24, 2015).
Crisafulli C, Fabbri C, Porcelli S, Drago A, Spina E, De Ronchi D, et al. Pharmacogenetics of antidepressants. Front Pharmacol 2011;2:6. http://dx.doi.org/10.3389/fphar.2011.00006.
Fornaro M, Giosuè P. Current nosology of treatment resistant depression: a controversy resistant to revision. Clin Pract Epidemiol Ment Health 2010;6:20–4. http://dx.doi.org/10.2174/1745017901006010020.
Pytka K, Podkowa K, Rapacz A, Podkowa A, Żmudzka E, Olczyk A, et al. The role of serotonergic, adrenergic and dopaminergic receptors in antidepressant-like effect. Pharmacol Rep 2015. http://dx.doi.org/10.1016/j.pharep.2015.08.007.
Chaki S, Ago Y, Palucha-Paniewiera A, Matrisciano F, Pilc A. mGlu2/3 and mGlu5 receptors: potential targets for novel antidepressants. Neuropharmacology 2013;66:40–52. http://dx.doi.org/10.1016/j.neuropharm.2012.05.022.
Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol 1997;37:205–37. http://dx.doi.org/10.1146/annurev.pharmtox.37.1.205.
Ferraguti F, Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res 2006;326:483–504. http://dx.doi.org/10.1007/s00441-006-0266-5.
Wierońska JM, Legutko B, Dudys D, Pilc A. Olfactory bulbectomy and amitriptyline treatment influences mGlu receptors expression in the mouse brain hippocampus. Pharmacol Rep 2008;60:844–55.
Matrisciano F, Caruso A, Orlando R, Marchiafava M, Bruno V, Battaglia G, et al. Defective group-II metaboropic glutamate receptors in the hippocampus of spontaneously depressed rats. Neuropharmacology 2008;55:525–31. http://dx.doi.org/10.1016/j.neuropharm.2008.05.014.
Matrisciano F, Storto M, Ngomba RT, Cappuccio I, Caricasole A, Scaccianoce S, et al. Imipramine treatment up-regulates the expression and function of mGlu2/3 metabotropic glutamate receptors in the rat hippocampus. Neuropharmacology 2002;42:1008–15.
Deschwanden A, Karolewicz B, Feyissa AM, Treyer V, Ametamey SM, Johayem A, et al. Reduced metabotropic glutamate receptor 5 density in major depression determined by [(11)C]ABP688 PET and postmortem study. Am J Psychiatry 2011;168:727–34. http://dx.doi.org/10.1176/appi.ajp.2011.09111607.
Feyissa AM, Woolverton WL, Miguel-Hidalgo JJ, Wang Z, Kyle PB, Hasler G, et al. Elevated level of metabotropic glutamate receptor 2/3 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry 2010;34:279–83. http://dx.doi.org/10.1016/j.pnpbp.2009.11.018.
Belozertseva IV, Kos T, Popik P, Danysz W, Bespalov AY. Antidepressant-like effects of mGluR1 and mGluR5 antagonists in the rat forced swim and the mouse tail suspension tests. Eur Neuropsychopharmacol 2007;17:172–9. http://dx.doi.org/10.1016/j.euroneuro.2006.03.002.
Pałucha A, Brański P, Szewczyk B, Wierońska JM, Kłak K, Pilc A. Potential antidepressant-like effect of MTEP, a potent and highly selective mGluR5 antagonist. Pharmacol Biochem Behav 2005;81:901–6. http://dx.doi.org/10.1016/j.pbb.2005.06.015.
Pałucha-Poniewiera A, Brański P, Wierońska JM, Stachowicz K, Sławińska A, Pilc A. The antidepressant-like action ofmGlu5 receptor antagonist, MTEP, in the tail suspension test in mice is serotonin dependent. Psychopharmacology 2014;231:97–107. http://dx.doi.org/10.1007/s00213-013-3206-6.
Domin H, Szewczyk B, Woźniak M, Wawrzak-Wleciał A, Śmiałowska M. Antidepressant-like effect of the mGluR5 antagonist MTEP in an astroglial degeneration model of depression. Behav Brain Res 2014;273:23–33. http://dx.doi.org/10.1016/j.bbr.2014.07.019.
Tatarczyńska E, Klodzińska A, Chojnacka-Wójcik E, Palucha A, Gasparini F, Kuhn R, et al. Potential anxiolytic- and antidepressant-like effects of MPEP, a potent, selective and systemically active mGlu5 receptor antagonist. Br J Pharmacol 2001;132:1423–30. http://dx.doi.org/10.1038/sj.bjp.0703923.
Wieronska JM, Szewczyk B, Branski P, Palucha A, Pilc A. Antidepressant-like effect of MPEP, a potent, selective and systemically active mGlu5 receptor antagonist in the olfactory bulbectomized rats. Amino Acids 2002;23:213–6. http://dx.doi.org/10.1007/s00726-001-0131-5.
Pilc A, Chaki S, Nowak G, Witkin JM. Mood disorders: regulation by metabotropic glutamate receptors. Biochem Pharmacol 2008;75:997–1006. http://dx.doi.org/10.1016/j.bcp.2007.09.021.
Bespalov AY, van Gaalen MM, Sukhotina IA, Wicke K, Mezler M, Schoemaker H, et al. Behavioral characterization of the mGlu group II/III receptor antagonist, LY-341495, in animal models of anxiety and depression. Eur J Pharmacol 2008;592:96–102. http://dx.doi.org/10.1016/j.ejphar.2008.06.089.
Chaki S, Yoshikawa R, Hirota S, Shimazaki T, Maeda M, Kawashima N, et al. MGS0039: a potent and selective group II metabotropic glutamate receptor antagonist with antidepressant-like activity. Neuropharmacology 2004;46:457–67. http://dx.doi.org/10.1016/j.neuropharm.2003.10.009.
Yoshimizu T, Shimazaki T, Ito A, Chaki S. An mGluR2/3 antagonist, MGS0039, exerts antidepressant and anxiolytic effects in behavioral models in rats. Psychopharmacology 2006;186:587–93. http://dx.doi.org/10.1007/s00213-006-0390-7.
Tatarczyńska E, Pałucha A, Szewczyk B, Chojnacka-Wójcik E, Wierońska J, Pilc A. Anxiolytic- and antidepressant-like effects of group III metabotropic glutamate agonist (1S,3R,4S)-1-aminocyclopentane-1,3,4-tricarboxylic acid (ACPT-1) in rats. Pol J Pharmacol 2002;54:707–10.
Pałucha A, Tatarczyńska E, Brański P, Szewczyk B, Wierońska JM, Kłak K, et al. Group III mGlu receptor agonists produce anxiolytic- and antidepressant-like effects after central administration in rats. Neuropharmacology 2004;46:151–9.
Wierońska JM, Stachowicz K, Pałucha-Poniewiera A, Acher F, Brański P, Pilc A. Metabotropic glutamate receptor 4 novel agonist LSP 1-2111 with anxiolytic, but not antidepressant-like activity, mediated by serotonergic and GABAergic systems. Neuropharmacology 2010;59:627–34. http://dx.doi.org/10.1016/j.neuropharm.2010.08.008.
Palucha A, Klak K, Branski P, van der Putten H, Flor PJ, Pilc A. Activation of the mGlu7 receptor elicits antidepressant-like effects in mice. Psychopharmacology 2007;194:555–62. http://dx.doi.org/10.1007/s00213-007-0856-2.
Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol 2001;11:327–35.
Malkesman O, Austin DR, Tragon T, Wang G, Rompala G, Hamidi AB, et al. Acute D-serine treatment produces antidepressant-like effects in rodents. Int J Neuropsychopharmacol 2012;15:1135–48. http://dx.doi.org/10.1017/S1461145711001386.
Młyniec K, Davies CL, de Agüero Sánchez IG, Pytka K, Budziszewska B, Nowak G. Essential elements in depression and anxiety. Part I. Pharmacol Rep 2014;66:534–44. http://dx.doi.org/10.1016/j.pharep.2014.03.001.
Nowak G, Trullas R, Layer RT, Skolnick P, Paul IA. Adaptive changes in the N-methyl-D-aspartate receptor complex after chronic treatment with imipramine and 1-aminocyclopropanecarboxylic acid. J Pharmacol Exp Ther 1993;265:1380–6.
Nowak G, Li Y, Paul IA. Adaptation of cortical but not hippocampal NMDA receptors after chronic citalopram treatment. Eur J Pharmacol 1996;295:75–85.
Nowak G, Legutko B, Skolnick P, Popik P. Adaptation of cortical NMDA receptors by chronic treatment with specific serotonin reuptake inhibitors. Eur J Pharmacol 1998;342:367–70.
Skolnick P. Antidepressants for the new millennium. Eur J Pharmacol 1999;375:31–40.
Boyer PA, Skolnick P, Fossom LH. Chronic administration of imipramine and citalopram alters the expression of NMDA receptor subunit mRNAs in mouse brain. A quantitative in situ hybridization study. J Mol Neurosci 1998;10:219–33. http://dx.doi.org/10.1007/BF02761776.
Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 2010;67:793–802. http://dx.doi.org/10.1001/archgenpsychiatry.2010.90.
Scarr E, Pavey G, Sundram S, MacKinnon A, Dean B. Decreased hippocampal NMDA, but not kainate or AMPA receptors in bipolar disorder. Bipolar Disord 2003;5:257–64.
Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry 2009;33:70–5. http://dx.doi.org/10.1016/j.pnpbp.2008.10.005.
Nudmamud-Thanoi S, Reynolds GP. The NR1 subunit of the glutamate/NMDA receptor in the superior temporal cortex in schizophrenia and affective disorders. Neurosci Lett 2004;372:173–7. http://dx.doi.org/10.1016/j.neulet.2004.09.035.
Karolewicz B, Szebeni K, Gilmore T, Maciag D, Stockmeier CA, Ordway GA. Elevated levels of NR2A and PSD-95 in the lateral amygdala in depression. Int J Neuropsychopharmacol 2009;12:143–53. http://dx.doi.org/10.1017/S1461145708008985.
Karolewicz B, Stockmeier CA, Ordway GA. Elevated levels of the NR2C subunit of the NMDA receptor in the locus coeruleus in depression. Neuropsychopharmacology 2005;30:1557–67. http://dx.doi.org/10.1038/sj.npp.1300781.
Trullas R, Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur J Pharmacol 1990;185:1–10.
Dang YH, Ma XC, Zhang JC, Ren Q, Wu J, Gao CG, et al. Targeting of NMDA receptors in the treatment of major depression. Curr Pharm Des 2014;20: 5151–5159.
Crane GE. Cyloserine as an antidepressant agent. Am J Psychiatry 1959;115:1025–1026.
Dravid SM, Burger PB, Prakash A, Geballe MT, Yadav R, Le P, et al. Structural determinants of D-cycloserine efficacy at the NR1/NR2 C NMDA receptors. J Neurosci 2010;30:2741–54. http://dx.doi.org/10.1523/JNEUROSC1.5390-09.2010.
Trullas R, Folio T, Young A, Miller R, Boje K, Skolnick P. 1-aminocyclopropanecarboxylates exhibit antidepressant and anxiolytic actions in animal models. Eur J Pharmacol 1991;203:379–85.
Burgdorf J, Zhang XL, Nicholson KL, Balster RL, Leander JD, Stanton PK, et al. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology 2013;38:729–42. http://dx.doi.org/10.1038/npp.2012.246.
Papp M, Moryl E. Similar effect of chronic treatment with imipramine and the NMDA antagonists CGP 37849 and MK-801 in a chronic mild stress model of depression in rats. Eur Neuropsychopharmacol 1993;3:348–9. http://dx.doi.org/10.1016/0924-977X(93)90130-E.
Papp M, Moryl E. Antidepressant activity of non-competitive and competitive NMDA receptor antagonists in a chronic mild stress model of depression. Eur J Pharmacol 1994;263:1–7.
Réus GZ, Stringari RB, Kirsch TR, Fries GR, Kapczinski F, Roesler R, et al. Neurochemical and behavioural effects of acute and chronic memantine administration in rats: further support for NMDA as a new pharmacological target for the treatment of depression? Brain Res Bull 2010;81:585–9. http://dx.doi.org/10.1016/j.brainresbull.2009.11.013.
Réus GZ, Abelaira HM, Stringari RB, Fries GR, Kapczinski F, Quevedo J. Memantine treatment reverses anhedonia, normalizes corticosterone levels and increases BDNF levels in the prefrontal cortex induced by chronic mild stress in rats. Metab Brain Dis 2012;27:175–82. http://dx.doi.org/10.1007/s11011-012-9281-2.
Quan MN, Zhang N, Wang YY, Zhang T, Yang Z. Possible antidepressant effects and mechanisms of memantine in behaviors and synaptic plasticity of a depression rat model. Neuroscience 2011;182:88–97. http://dx.doi.org/10.1016/j.neuroscience.2011.03.026.
Huber T, Dietrich D, Emrich H. Possible use of amantadine in depression. Pharmacopsychiatry 2007;32:47–55. http://dx.doi.org/10.1055/s-2007-979191.
Ossowska G, Klenk-Majewska B, Szymczyk G. The effect of NMD Aantagonists on footshock-induced fighting behavior in chronically stressed rats. J Physiol Pharmacol 1997;48:127–35.
Redmond AM, Kelly JP, Leonard BE. Behavioural neurochemical effects of dizocilpine in the olfactory bulbectomized rat model of depression. Pharmacol Biochem Behav 1997;58(2):355–9.
Yilmaz A, Schulz D, Aksoy A, Canbeyli R. Prolonged effect of an anesthetic dose of ketamine on behavioral despair. Pharmacol Biochem Behav 2002;71:341–4.
Garcia LS, Comim CM, Valvassori SS, Réus GZ, Andreazza AC, Stertz L, et al. Chronic administration of ketamine elicits antidepressant-like effects in rats without affecting hippocampal brain-derived neurotrophic factor protein levels. Basic Clin Pharmacol Toxicol 2008;103:502–6. http://dx.doi.org/10.1111/j.1742-7843.2008.00210.x.
Garcia LSB, Comim CM, Valvassori SS, Réus GZ, Barbosa LM, Andreazza AC, et al. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry 2008;32:140–4. http://dx.doi.org/10.1016/j.pnpbp.2007.07.027.
Garcia LSB, Comim CM, Valvassori SS, Réus GZ, Stertz L, Kapczinski F, et al. Ketamine treatment reverses behavioral and physiological alterations induced by chronic mild stress in rats. Prog Neuropsychopharmacol Biol Psychiatry 2009;33:450–5. http://dx.doi.org/10.1016/j.pnpbp.2009.01.004.
Gideons ES, Kavalali ET, Monteggia LM. Mechanisms underlying differential effectiveness of memantine and ketamine in rapid antidepressant responses. Proc Natl Acad Sci U S A 2014;111:8649–54. http://dx.doi.org/10.1073/pnas.1323920111.
Młyniec K, Gaweł M, Doboszewska U, Starowicz G, Pytka K, Davies CL, et al. Essential elements in depression and anxiety. Part II. Pharmacol Rep 2015;67:187–94. http://dx.doi.org/10.1016/j.pharep.2014.09.009.
Mlyniec K. Zinc in the Glutamatergic Theory of depression. Curr Neuropharmacol 2015;13. http://dx.doi.org/10.2174/1570159X13666150115220617. 1–1.
Maes M, De Vos N, Demedts P, Wauters A, Neels H. Lower serum zinc in major depression in relation to changes in serum acute phase proteins. J Affect Disord 1999;56:189–94. http://dx.doi.org/10.1016/S0165-0327(99)00011-7.
McLoughlin IJ, Hodge JS. Zinc in depressive disorder. Acta Psychiatr Scand 1990;82:451–3.
Swardfager W, Herrmann N, Mazereeuw G, Goldberger K, Harimoto T, Lanctôt KL. Zinc in depression: a meta-analysis. Biol Psychiatry 2013;74:872–8. http://dx.doi.org/10.1016/j.biopsych.2013.05.008.
Doboszewska U, Sowa-Kućma M, Młyniec K, Pochwat B, Hołuj M, Ostachowicz B, et al. Zinc deficiency in rats is associated with up-regulation of hippocampal NMDA receptor. Prog Neuropsychopharmacol Biol Psychiatry 2015;56:254–63. http://dx.doi.org/10.1016/j.pnpbp.2014.09.013.
Młyniec K, Nowak G. Zinc deficiency induces behavioral alterations in the tail suspension test in mice. Effect of antidepressants. Pharmacol Rep 2012;64:249–255.
Młyniec K, Budziszewska B, Reczyński W, Doboszewska U, Pilc A, Nowak G. Zinc deficiency alters responsiveness to antidepressant drugs in mice. Pharmacol Rep 2013;65:579–92.
Młyniec K, Doboszewska U, Szewczyk B, Sowa-Kućma M, Misztak P, Piekoszewski W, et al. The involvement of the GPR39-Zn(2+)-sensing receptor in the pathophysiology of depression. Studies in rodent models and suicide victims. Neuropharmacology 2014;79C:290–7. http://dx.doi.org/10.1016/j.neuropharm.2013.12.001.
Młyniec K, Davies CL, Budziszewska B, Opoka W, Reczyński W, Sowa-Kućma M, et al. Time course of zinc deprivation-induced alterations of mice behavior in the forced swim test. Pharmacol Rep 2012;64:567–75.
Tassabehji NM, Corniola RS, Alshingiti A, Levenson CW. Zinc deficiency induces depression-like symptoms in adult rats. Physiol Behav 2008;95:365–9. http://dx.doi.org/10.1016/j.physbeh.2008.06.017.
Whittle N, Lubec G, Singewald N. Zinc deficiency induces enhanced depression-like behaviour and altered limbic activation reversed by antidepressant treatment in mice. Amino Acids 2009;36:147–58. http://dx.doi.org/10.1007/s00726-008-0195-6.
Holst B, Egerod KL, Schild E, Vickers SP, Cheetham S, Gerlach L-O, et al. GPR39 signaling is stimulated by zinc ions but not by obestatin. Endocrinology 2007;148:13–20. http://dx.doi.org/10.1210/en.2006–0933.
Młyniec K, Singewald N, Holst B, Nowak G. GPR39 Zn2+ -sensing receptor: a new target in antidepressant development? J Affect Disord 2015;174:89–100. http://dx.doi.org/10.1016/j.jad.2014.11.033.
Młyniec K, Budziszewska B, Holst B, Ostachowicz B, Nowak G. GPR39 (zinc receptor) knockout mice exhibit depression-like behavior and CREB/BDNF down-regulation in the hippocampus. Int J Neuropsychopharmacol 2015;18:1–8. http://dx.doi.org/10.1093/ijnp/pyu002.
Młyniec K, Gaweł M, Nowak G. Study of antidepressant drugs in GPR39 (zinc receptor−/−) knockout mice, showing no effect of conventional antidepressants, but effectiveness of NMDA antagonists. Behav Brain Res 2015;287:135–8. http://dx.doi.org/10.1016/j.bbr.2015.03.053.
Eby Ga, Eby KL. Rapid recovery from major depression using magnesium treatment. Med Hypotheses 2006;67:362–70. http://dx.doi.org/10.1016/j.mehy.2006.01.047.
Poleszak E, Wlaź P, Szewczyk B, Kedzierska E, Wyska E, Librowski T, et al. Enhancement of antidepressant-like activity by joint administration of imipramine and magnesium in the forced swim test: behavioral and pharmacokinetic studies in mice. Pharmacol Biochem Behav 2005;81:524–9. http://dx.doi.org/10.1016/j.pbb.2005.03.017.
Poleszak E, Wlaź P, Kedzierska E, Nieoczym D, Wróbel A, Fidecka S, et al. NMDA/glutamate mechanism of antidepressant-like action ofmagnesium in forced swim test in mice. Pharmacol Biochem Behav 2007;88:158–64. http://dx.doi.org/10.1016/j.pbb.2007.07.018.
Pochwat B, Szewczyk B, Sowa-Kucma M, Siwek A, Doboszewska U, Piekoszewski W, et al. Antidepressant-like activity of magnesium in the chronic mild stress model in rats: alterations in the NMDA receptor subunits. Int J Neuropsychopharmacol 2014;17:393–405. http://dx.doi.org/10.1017/S1461145713001089.
Pochwat B, Sowa-Kucma M, Kotarska K, Misztak P, Nowak G, Szewczyk B. Antidepressant-like activity of magnesium in the olfactory bulbectomy model is associated with the AMPA/BDNF pathway. Psychopharmacology 2015;232:355–67. http://dx.doi.org/10.1007/s00213-014-3671-6.
Singewald N, Sinner C, Hetzenauer A, Sartori SB, Murck H. Magnesium-deficient diet alters depression- and anxiety-related behavior in mice—influence of desipramine and Hypericum perforatum extract. Neuropharmacology 2004;47:1189–97. http://dx.doi.org/10.1016/j.neuropharm.2004.08.010.
Sartori SB, Whittle N, Hetzenauer A, Singewald N. Magnesium deficiency induces anxiety and HPA axis dysregulation: modulation by therapeutic drug treatment. Neuropharmacology 2012;62:304–12. http://dx.doi.org/10.1016/j.neuropharm.2011.07.027.
Salari A-A, Bakhtiari A, Homberg JR. Activation of GABA-A receptors during postnatal brain development increases anxiety- and depression-related behaviors in a time- and dose-dependent manner in adult mice. Eur Neuropsychopharmacol 2015. http://dx.doi.org/10.1016/j.euroneuro.2015.04.022.
Pehrson AL, Altered Sanchez C. γ-aminobutyric acid neurotransmission in major depressive disorder: a critical review of the supporting evidence and the influence of serotonergic antidepressants. Drug Des Devel Ther 2015;9:603–24. http://dx.doi.org/10.2147/DDDT.S62912.
Frankowska M, Filip M, Przegaliński E. Effects of GABAB receptor ligands in animal tests of depression and anxiety. Pharmacol Rep 2007;59:645–55.
Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, et al. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev 1998;50:291–313.
Sigel E, Steinmann ME. Structure, function, and modulation of GABA(A) receptors. J Biol Chem 2012;287:40224–31. http://dx.doi.org/10.1074/jbc.R112.386664.
Brambilla P, Perez J, Barale F, Schettini G, Soares JC. GABAergic dysfunction in mood disorders. Mol Psychiatry 2003;8:721–37. http://dx.doi.org/10.1038/sj.mp.4001362.715.
Bowery NG, Bettler B, Froestl W, Gallagher JP, Marshall F, Raiteri M, et al. International Union of Pharmacology. XXXIII. Mammalian gamma-amino-butyric acid(B) receptors: structure and function. Pharmacol Rev 2002;54:247–64.
McDonald AJ, Mascagni F, Muller JF. Immunocytochemical localization of GABABR1 receptor subunits in the basolateral amygdala. Brain Res 2004;1018:147–58. http://dx.doi.org/10.1016/j.brainres.2004.05.053.
Gold BI, Bowers MB, Roth RH, Sweeney DW. GABA levels in CSF of patients with psychiatric disorders. Am J Psychiatry 1980;137:362–4.
Möhler H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology 2012;62:42–53. http://dx.doi.org/10.1016/j.neuropharm.2011.08.040.
Thoeringer CK, Ripke S, Unschuld PG, Lucae S, Ising M, Bettecken T, et al. The GABA transporter 1 (SLC6A1): a novel candidate gene for anxiety disorders. J Neural Transm 2009;116:649–57. http://dx.doi.org/10.1007/s00702-008-0075-y.
Karolewicz B, Maciag D, O’Dwyer G, Stockmeier CA, Feyissa AM, Rajkowska G. Reduced level of glutamic acid decarboxylase-67 kDa in the prefrontal cortex in major depression. Int J Neuropsychopharmacol 2010;13:411–20. http://dx.doi.org/10.1017/S1461145709990587.
Sanacora G, Gueorguieva R, Epperson CN, Wu Y-T, Appel M, Rothman DL, et al. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry 2004;61:705–13. http://dx.doi.org/10.1001/archpsyc.61.7.705.
Rajkowska G, O’Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology 2007;32:471–82. http://dx.doi.org/10.1038/sj.npp.1301234.
Guilloux J-P, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, et al. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry 2012;17:1130–42. http://dx.doi.org/10.1038/mp.2011.113.
Tripp A, Kota RS, Lewis DA, Sibille E. Reduced somatostatin in subgenual anterior cingulate cortex in major depression. Neurobiol Dis 2011;42: 116–124. http://dx.doi.org/10.1016/j.nbd.2011.01.014.
Engin E, Liu J, Rudolph U. α2-containing GABA(A) receptors: a target for the development of novel treatment strategies for CNS disorders. Pharmacol Ther 2012;136:142–52. http://dx.doi.org/10.1016/j.pharmthera.2012.08.006.
Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry 2011;16:383–406. http://dx.doi.org/10.1038/mp.2010.120.
Earnheart JC, Schweizer C, Crestani F, Iwasato T, Itohara S, Mohler H, et al. GABAergic control of adult hippocampal neurogenesis in relation to behavior indicative of trait anxiety and depression states. J Neurosci 2007;27:3845–3854. http://dx.doi.org/10.1523/JNEUROSCI. 3609-06.2007.
Vollenweider I, Smith KS, Keist R, Rudolph U. Antidepressant-like properties of α2-containing GABa(a) receptors. Behav Brain Res 2011;217:77–80. http://dx.doi.org/10.1016/j.bbr.2010.10.009.
Fava M, McCall WV, Krystal A, Wessel T, Rubens R, Caron J, et al. Eszopiclone co-administered with fluoxetine in patients with insomnia coexisting with major depressive disorder. Biol Psychiatry 2006;59:1052–60. http://dx.doi.org/10.1016/j.biopsych.2006.01.016.
Fava M, Schaefer K, Huang H, Wilson A, Iosifescu DV, Mischoulon D, et al. A post hoc analysis of the effect of nightly administration of eszopiclone and a selective serotonin reuptake inhibitor in patients with insomnia and anxious depression. J Clin Psychiatry 2011;72:473–9. http://dx.doi.org/10.4088/JCP.09m05131gry.
Krystal A, Fava M, Rubens R, Wessel T, Caron J, Wilson P, et al. Evaluation of eszopiclone discontinuation after cotherapy with fluoxetine for insomnia with coexisting depression. J Clin Sleep Med 2007;3:48–55.
Samardžić J, Puškaš L, Obradović M, Lazić-Puškaš D. Antidepressant effects of an inverse agonist selective for α5 GABA-A receptors in the rat forced swim test. Acta Vet Brno 2014;64:52–60. http://dx.doi.org/10.2478/acve-2014-0006.
Pilc A, Nowak G. GABAergic hypotheses of anxiety and depression: focus on GABA-B receptors. Drugs Today 2005;41:755–66. http://dx.doi.org/10.1358/dot.2005.41.11.904728.
Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry 2002;159:663–5.
Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci 2002;23:238–45.
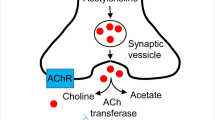
Janowsky DS, El-Yousef MK, Davis JM, Sekerke HJ. A cholinergic-adrenergic hypothesis of mania and depression. Lancet 1972;2:632–5.
Wess J. Novel insights into muscarinic acetylcholine receptor function using gene targeting technology. Trends Pharmacol Sci 2003;24:414–20. http://dx.doi.org/10.1016/S0165-6147(03)00195-0.
Bertrand D. The possible contribution of neuronal nicotinic acetylcholine receptors in depression. Dialogues Clin Neurosci 2005;7:207–16.
Bymaster FP, Felder CC. Role of the cholinergic muscarinic system in bipolar disorder and related mechanism of action of antipsychotic agents. Mol Psychiatry 2002;7(Suppl 1):S57–63. http://dx.doi.org/10.1038/sj.mp.4001019.
Role LW, Berg DK. Nicotinic receptors in the development and modulation of CNS synapses. Neuron 1996;16:1077–85.
Graham A, Court JA, Martin-Ruiz CM, Jaros E, Perry R, Volsen SG, et al. Immunohistochemical localisation of nicotinic acetylcholine receptor sub-units in human cerebellum. Neuroscience 2002;113:493–507.
Dani JA. Overview of nicotinic receptors and their roles in the central nervous system. Biol Psychiatry 2001;49:166–74. http://dx.doi.org/10.1016/S0006-3223(00)01011-8.
Swanson LW, Simmons DM, Whiting PJ, Lindstrom J. Immunohistochemical localization of neuronal nicotinic receptors in the rodent central nervous system. J Neurosci 1987;7:3334–42.
Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci 2006;27:482–91. http://dx.doi.org/10.1016/j.tips.2006.07.004.
Gotti C, Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol 2004;74:363–96. http://dx.doi.org/10.1016/j.pneurobio.2004.09.006.
Broadley KJ, Kelly DR. Muscarinic Receptor Agonists and Antagonists. Molecules 2001;6:142–93. http://dx.doi.org/10.3390/60300142.
Levey AI. Immunological localization of m1-m5 muscarinic acetylcholine receptors in peripheral tissues and brain. Life Sci 1993;52:441–8.
Gomeza J, Shannon H, Kostenis E, Felder C, Zhang L, Brodkin J, et al. Pronounced pharmacologic deficits in M2 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci U S A 1999;96:1692–7.
Hemrick-Luecke SK, Bymaster FP, Evans DC, Wess J, Felder CC. Muscarinic agonist-mediated increases in serum corticosterone levels are abolished in m(2) muscarinic acetylcholine receptor knockout mice. J Pharmacol Exp Ther 2002;303:99–103. http://dx.doi.org/10.1124/jpet.102.036020.
Bymaster FP, McKinzie DL, Felder CC, Wess J. Use of M1-M5 muscarinic receptor knockout mice as novel tools to delineate the physiological roles of the muscarinic cholinergic system. Neurochem Res 2003;28:437–42.
Korbut R. Farmakologia. Warszawa: PZWL; 2014.
Steingard RJ, Yurgelun-Todd DA, Hennen J, Moore JC, Moore CM, Vakili K, et al. Increased orbitofrontal cortex levels of choline in depressed adolescents as detected by in vivo proton magnetic resonance spectroscopy. Biol Psychiatry 2000;48:1053–61.
Meyerson LR, Wennogle LP, Abel MS, Coupet J, Lippa AS, Rauh CE, et al. Human brain receptor alterations in suicide victims. Pharmacol Biochem Behav 1982;17:159–63.
Gibbons DL, Lin W, Creighton CJ, Rizvi ZH, Gregory PA, Goodall GJ, et al. Contextual extracellular cues promote tumor cell EMT and metastasis by regulating miR-200 family expression. Genes Dev 2009;23:2140–51. http://dx.doi.org/10.1101/gad.1820209.
Chau DT, Rada P, Kosloff RA, Taylor JL, Hoebel BG. Nucleus accumbens muscarinic receptors in the control of behavioral depression: antidepressant-like effects of local M1 antagonist in the Porsolt swim test. Neuroscience 2001;104:791–8.
Witkin JM, Overshiner C, Li X, Catlow JT, Wishart GN, Schober DA, et al. M1 and M2 muscarinic receptor subtypes regulate antidepressant-like effects of the rapidly acting antidepressant scopolamine. J Pharmacol Exp Ther 2014;351:448–56. http://dx.doi.org/10.1124/jpet.114.216804.
Shioda N, Yamamoto Y, Han F, Moriguchi S, Yamaguchi Y, Hino M, et al. A novel cognitive enhancer, ZSET1446/ST101, promotes hippocampal neurogenesis and ameliorates depressive behavior in olfactory bulbectomized mice. J Pharmacol Exp Ther 2010;333:43–50. http://dx.doi.org/10.1124/jpet.109.163535.
Mineur YS, Eibl C, Young G, Kochevar C, Papke RL, Gündisch D, et al. Cytisine-based nicotinic partial agonists as novel antidepressant compounds. J Pharmacol Exp Ther 2009;329:377–86. http://dx.doi.org/10.1124/jpet.108.149609.
Caldarone BJ, Wang D, Paterson NE, Manzano M, Fedolak A, Cavino K, et al. Dissociation between duration of action in the forced swim test in mice and nicotinic acetylcholine receptor occupancy with sazetidine, varenicline, and 5-1-A85380. Psychopharmacology 2011;217:199–210. http://dx.doi.org/10.1007/s00213-011-2271-y.
**ao Y, Fan H, Musachio JL, Wei Z-L, Chellappan SK, Kozikowski AP, et al. Sazetidine-A, a novel ligand that desensitizes alpha4beta2 nicotinic acetylcholine receptors without activating them. Mol Pharmacol 2006;70:1454–60. http://dx.doi.org/10.1124/mol.106.027318.
Buckley MJ, Surowy C, Meyer M, Curzon P. Mechanism ofaction of A-85380 in an animal model of depression. Prog Neuropsychopharmacol Biol Psychiatry 2004;28:723–30. http://dx.doi.org/10.1016/j.pnpbp.2004.05.012.
Rollema H, Guanowsky V, Mineur YS, Shrikhande A, Coe JW, Seymour PA, et al. Varenicline has antidepressant-like activity in the forced swim test and augments sertraline’s effect. Eur J Pharmacol 2009;605:114–6. http://dx.doi.org/10.1016/j.ejphar.2009.01.002.
Gatto GJ, Bohme GA, Caldwell WS, Letchworth SR, Traina VM, Obinu MC, et al. TC-1734: an orally active neuronal nicotinic acetylcholine receptor modulator with antidepressant, neuroprotective and long-lasting cognitive effects. CNS Drug Rev 2004;10:147–66.
Ferguson SM, Brodkin JD, Lloyd GK, Menzaghi F. Antidepressant-like effects of the subtype-selective nicotinic acetylcholine receptor agonist, SIB-1508Y, in the learned helplessness rat model of depression. Psychopharmacology 2000;152:295–303.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pytka, K., Dziubina, A., Młyniec, K. et al. The role of glutamatergic, GABA-ergic, and cholinergic receptors in depression and antidepressant-like effect. Pharmacol. Rep 68, 443–450 (2016). https://doi.org/10.1016/j.pharep.2015.10.006
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.pharep.2015.10.006