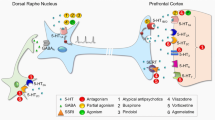
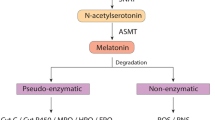
Abstract
Depression is a serious global illness, becoming more and more common in developed countries. Because of specific symptoms it is considered as a leading cause of disability all over the world with a high death factor due to suicides. There are many antidepressants used in the therapy, but still more than 30% of patients do not respond to the treatment. The heterogeneous nature of the illness and its complex, unclear aetiology may be responsible for these difficulties. Next to the main monoaminergic hypothesis of depression there are also many other approaches connected with the pathophysiology of the disease, including hypothalamic-pituitary-adrenal axis dysregulation, dopaminergic, cholinergic, glutamatergic or GABA-ergic neurotransmission. Nevertheless, it can be unambiguously stated that serotonergic, noradrenergic and dopaminergic systems are precisely connected with pathogenesis of depression, and should be therefore considered as valuable targets in patients’ treatment. Bearing that in mind, this review presents the role of serotonergic, adrenergic and dopaminergic receptors in antidepressant-like effect.
Similar content being viewed by others
References
WHO. Depression n.d. http://www.who.int/topics/depression/en/ [accessed 24.05.15].
Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry 1965;122:509–22.
Slattery DA, Cryan JF. The ups and downs of modelling mood disorders in rodents. ILAR J 2014;55:297–309. http://dx.doi.org/10.1093/ilar/ilu026.
Mark GA, Markou A. Animal models of psychiatric disorders. New York: Raven Press; 1995.
Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev 2005;29:571–625. http://dx.doi.org/10.1016/j.neubiorev.2005.03.009.
Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology (Berl) 2005;177:245–55. http://dx.doi.org/10.1007/s00213-004-2048-7.
Pytka K, Rapacz A, Zygmunt M, Olczyk A, Waszkielewicz A, Sapa J, et al. Antidepressant-like activity of a new piperazine derivative of xanthone in the forced swim test in mice: the involvement of serotonergic system. Pharmacol Rep 2015;67:160–5. http://dx.doi.org/10.1016/j.pharep.2014.08.016.
Pytka K, Walczak M, Kij A, Rapacz A, Siwek A, Kazek G, et al. The antidepressant-like activity of 6-methoxy-2-[4-(2-methoxyphenyl)piperazin-1-yl]-9H-xanthen-9-one involves serotonergic 5-HT1A and 5-HT2A/C receptors activation. Eur J Pharmacol 2015. http://dx.doi.org/10.1016/j.ejphar.2015.07.041.
Bourin M, Mocaër E, Porsolt R. Antidepressant-like activity of S 20098 (agomelatine) in the forced swimming test in rodents: involvement of melatonin and serotonin receptors. J Psychiatry Neurosci 2004;29:126–33.
Berrocoso E, Ikeda K, Sora I, Uhl GR, Sánchez-Blázquez P, Mico JA. Active behaviours produced by antidepressants and opioids in the mouse tail suspension test. Int J Neuropsychopharmacol 2013;16:151–62. http://dx.doi.org/10.1017/S1461145711001842.
Kuśmider M, Solich J, Pałach P, Dziedzicka-Wasylewska M. Effect of citalopram in the modified forced swim test in rats. Pharmacol Rep 2007;59:785–8.
Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Thér 1977;229:327–36.
Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol 1978;47:379–91.
Borsini F. Role of the serotonergic system in the forced swimming test. Neurosci Biobehav Rev 1995;19:377–95.
Maj J, Rogóz Z, Skuza G, Sowińska H. Effects of MK-801 and antidepressant drugs in the forced swimming test in rats. Eur Neuropsychopharmacol 1992;2:37–41.
Górka Z, Wojtasik E, Kwiatek H, Maj J. Action of serotoninmimetics in the behavioral despair test in rats. Commun Psychopharmacol 1979;3:133–6.
Yamamoto T, Shibata S, Shimazoe T, Iwasaki K, Ohno M, Minamoto Y, et al. Behavioral pharmacological properties of the novel antidepressant paroxetine, a selective 5-HT uptake inhibitor. Nihon Yakurigaku Zasshi 1989;94:189–206.
Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72.
Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–70.
Carr GV, Lucki I. The role of serotonin receptor subtypes in treating depression: a review of animal studies. Psychopharmacology (Berl) 2010;213:265–87. http://dx.doi.org/10.1007/s00213-010-2097-z.
Mombereau C, Kaupmann K, Froestl W, Sansig G, van der Putten H, Cryan JF. Genetic and pharmacological evidence of a role for GABA(B) receptors in the modulation of anxiety- and antidepressant-like behavior. Neuropsychopharmacology 2004;29:1050–62. http://dx.doi.org/10.1038/sj.npp.1300413.
Koch M. Animal models of neuropsychiatric diseases, animal models of depression. London: Imperial College Press; 2006.
Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134:319–29.
Müller CP, Jacobs BL. Handbook of the behavioral neurobiology of serotonin, the role of serotonin in depression. London: Academic Press; 2010.
Czéh B, Fuchs E, Wiborg O, Simon M. Animal models of major depression and their clinical implications. Prog Neuropsychopharmacol Biol Psychiatry 2015. http://dx.doi.org/10.1016/j.pnpbp.2015.04.004.
Overstreet DH, Wegener G. The Flinders sensitive line rat model of depression — 25 years and still producing. Pharmacol Rev 2013;65:143–55. http://dx.doi.org/10.1124/pr.111.005397.
Overstreet DH. Modeling depression in animal models. Methods Mol Biol 2012;829:125–44. http://dx.doi.org/10.1007/978-1-61779-458-2_7.
Tsai H-C, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 2009;324:1080–4. http://dx.doi.org/10.1126/science.1168878.
Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 2011;475:377–80. http://dx.doi.org/10.1038/nature10194.
Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai H-C, Finkelstein J, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 2013;493:537–41. http://dx.doi.org/10.1038/nature11740.
Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim S-Y, et al. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature 2012;492:428–32. http://dx.doi.org/10.1038/nature11617.
Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature 2007;450:420–4. http://dx.doi.org/10.1038/nature06310.
McKim WA. Antidepressants and mood stabilizers. In: Drugs and behavior. New Jersey: Pearson, Prentice Hall; 2007.
Azmitia EC. Serotonin neurons, neuroplasticity, and homeostasis of neural tissue. Neuropsychopharmacology 1999;21:33S–45S. http://dx.doi.org/10.1016/S0893-133X(99)00022-6.
Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology 1999;38:1083–152.
Gaddum JH, Picarelli ZP. Two kinds of tryptamine receptor. Br J Pharmacol 1997;120:133–4.
Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain Res Mol Brain Res 1994;23:163–78.
Fargin A, Raymond JR, Lohse MJ, Kobilka BK, Caron MG, Lefkowitz RJ. The genomic clone G-21 which resembles a beta-adrenergic receptor sequence encodes the 5-HT1A receptor. Nature 1988;335:358–60. http://dx.doi.org/10.1038/335358a0.
Ito H, Halldin C, Farde L. Localization of 5-HT1A receptors in the living human brain using [carbonyl-11C]WAY-100635: PET with anatomic standardization technique. J Nucl Med 1999;40:102–9.
Nowak JZ, Zawilska JB. Receptors: structure, chracteristics, function. Warszawa: Wydawnictwo Naukowe PWN; 1997 [in Polish].
Gozlan H, El Mestikawy S, Pichat L, Glowinski J, Hamon M. Identification of presynaptic serotonin autoreceptors using a new ligand: 3H-PAT. Nature 1983;305:140–2.
Varnäs K, Halldin C, Hall H. Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Hum Brain Mapp 2004;22:246–60. http://dx.doi.org/10.1002/hbm.20035.
Fox AW. Onset of effect of 5-HT1B/1D agonists: a model with pharmacokinetic validation. Headache 2004;44:142–7.
Cryan JF, Lucki I. Antidepressant-like behavioral effects mediated by 5-hydroxytryptamine(2C) receptors. J Pharmacol Exp Ther 2000;295:1120–6.
Brunton LB, Lazo JS, Parker KL. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill; 2011.
Jensen AA, Davies PA, Bräuner-Osborne H, Krzywkowski K. 3B but which 3B and that’s just one of the questions: the heterogeneity of human 5-HT3 receptors. Trends Pharmacol Sci 2008;29:437–44. http://dx.doi.org/10.1016/j.tips.2008.06.001.
Meyers NL, Hickling RI. Pharmacology and metabolism of renzapride: a novel therapeutic agent for the potential treatment of irritable bowel syndrome. Drugs R D 2008;9:37–63.
Monsma FJ, Shen Y, Ward RP, Hamblin MW, Sibley DR. Cloning and expression of a novel serotonin receptor with high affinity for tricyclic psychotropic drugs. Mol Pharmacol 1993;43:320–7.
Mann JJ. Neurobiology of suicidal behaviour. Nat Rev Neurosci 2003;4:819–28. http://dx.doi.org/10.1038/nrn1220.
Asberg M, Träskman L, Thorén P. 5-HIAA in the cerebrospinal fluid. A biochemical suicide predictor? Arch Gen Psychiatry 1976;33:1193–7.
Mann JJ, Arango V, Marzuk PM, Theccanat S, Reis DJ. Evidence for the 5-HT hypothesis of suicide. A review of post-mortem studies. Br J Psychiatry Suppl 1989;7–14.
Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, et al. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology 2001;25:892–903. http://dx.doi.org/10.1016/S0893-133X(01)00310-4.
Mann JJ, Malone KM. Cerebrospinal fluid amines and higher-lethality suicide attempts in depressed inpatients. Biol Psychiatry 1997;41:162–71.
Coccaro EF, Siever LJ, Klar HM, Maurer G, Cochrane K, Cooper TB, et al. Serotonergic studies in patients with affective and personality disorders, correlates with suicidal and impulsive aggressive behavior. Arch Gen Psychiatry 1989;46:587–99.
Dulchin MC, Oquendo MA, Malone KM, Ellis SP, Li S, Mann JJ. Prolactin response to dl-fenfluramine challenge before and after treatment with paroxetine. Neuropsychopharmacology 2001;25:395–401. http://dx.doi.org/10.1016/S0893-133X(01)00239-1.
Dillon KA, Gross-Isseroff R, Israeli M, Biegon A. Autoradiographic analysis of serotonin 5-HT1A receptor binding in the human brain postmortem: effects of age and alcohol. Brain Res 1991;554:56–64.
Parsey RV, Oquendo MA, Simpson NR, Ogden RT, Van Heertum R, Arango V, et al. Effects of sex, age, and aggressive traits in man on brain serotonin 5-HT1A receptor binding potential measured by PET using [C-11]WAY-100635. Brain Res 2002;954:173–82.
Turecki G. The molecular bases of the suicidal brain. Nat Rev Neurosci 2014;15:802–16. http://dx.doi.org/10.1038/nrn3839.
Stockmeier CA. Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. J Psychiatr Res 2003;37:357–73.
Hirvonen J, Karlsson H, Kajander J, Lepola A, Markkula J, Rasi-Hakala H, et al. Decreased brain serotonin 5-HT1A receptor availability in medication-naive patients with major depressive disorder: an in-vivo imaging study using PET and [carbonyl-11C]WAY-100635. Int J Neuropsychopharmacol 2008;11:465–76. http://dx.doi.org/10.1017/S1461145707008140.
Cryan JF, Redmond AM, Kelly JP, Leonard BE. The effects of the 5-HT1A agonist flesinoxan, in three paradigms for assessing antidepressant potential in the rat. Eur Neuropsychopharmacol 1997;7:109–14.
Przegaliński E, Moryl E, Papp M. The effect of 5-HT1A receptor ligands in a chronic mild stress model of depression. Neuropharmacology 1995;34: 1305–10.
Singh A, Lucki I. Antidepressant-like activity of compounds with varying efficacy at 5-HT1A receptors. Neuropharmacology 1993;32:331–40.
Piñeyro G, Blier P. Autoregulation of serotonin neurons: role in antidepressant drug action. Pharmacol Rev 1999;51:533–91.
Artigas F, Romero L, de Montigny C, Blier P. Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci 1996;19:378–83. http://dx.doi.org/10.1016/S0166-2236(96)10037-0.
Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev 2005;29:547–69. http://dx.doi.org/10.1016/j.neubiorev.2005.03.008.
Scorza MC, Lladó-Pelfort L, Oller S, Cortés R, Puigdemont D, Portella MJ, et al. Preclinical and clinical characterization of the selective 5-HT(1A) receptor antagonist DU-125530 for antidepressant treatment. Br J Pharmacol 2012;167:1021–34. http://dx.doi.org/10.1111/j.1476-5381.2011.01770.x.
Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proc Natl Acad Sci USA 1998;95:10734–39.
Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, et al. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci USA 1998;95:14476–81.
Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, et al. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci USA 1998;95:15049–54.
Nishi K, Kanemaru K, Diksic M. A genetic rat model of depression, Flinders sensitive line, has a lower density of 5-HT(1A) receptors, but a higher density of 5-HT(1B) receptors, compared to control rats. Neurochem Int 2009;54:299–307. http://dx.doi.org/10.1016/j.neuint.2008.12.011.
Tatarczyńska E, Kłodzińska A, Stachowicz K, Chojnacka-Wójcik E. Effect of combined administration of 5-HT1A or 5-HT1B/1D receptor antagonists and antidepressants in the forced swimming test. Eur J Pharmacol 2004;487:133–42. http://dx.doi.org/10.1016/j.ejphar.2004.01.008.
Dawson LA, Hughes ZA, Starr KR, Storey JD, Bettelini L, Bacchi F, et al. Characterisation of the selective 5-HT1B receptor antagonist SB-616234-A (1-[6-(cis-3,5-dimethylpiperazin-1-yl)-2,3-dihydro-5-methoxyindol-1-yl]-1-[2′-methyl-4′-(5-methyl-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]methanone hydrochloride): in vivo neurochemical a. Neuropharmacology 2006;50:975–83 http://dx.doi.org/10.1016/j.neuropharm.2006.01.010.
Cervo L, Grignaschi G, Nowakowska E, Samanin R. 1-(3-Trifluoromethylphenyl) piperazine (TFMPP) in the ventral tegmental area reduces the effect of desipramine in the forced swimming test in rats: possible role of serotonin receptors. Eur J Pharmacol 1989;171:119–25.
Neumaier JF, Root DC, Hamblin MW. Chronic fluoxetine reduces serotonin transporter mRNA and 5-HT1B mRNA in a sequential manner in the rat dorsal raphe nucleus. Neuropsychopharmacology 1996;15:515–22. http://dx.doi.org/10.1016/S0893-133X(96)00095-4.
Saudou F, Amara DA, Dierich A, LeMeur M, Ramboz S, Segu L, et al. Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science 1994;265:1875–8.
Trillat AC, Malagié I, Bourin M, Jacquot C, Hen R, Gardier AM. Homozygote mice deficient in serotonin 5-HT1B receptor and antidepressant effect of selective serotonin reuptake inhibitors. C R Seances Soc Biol Fil 1998;192:1139–47.
Jones MD, Lucki I. Sex differences in the regulation of serotonergic transmission and behavior in 5-HT receptor knockout mice. Neuropsychopharmacology 2005;30:1039–47. http://dx.doi.org/10.1038/sj.npp.1300664.
Patel JG, Bartoszyk GD, Edwards E, Ashby CR. The highly selective 5-hydroxytryptamine (5-HT)2A receptor antagonist, EMD 281014, significantly increases swimming and decreases immobility in male congenital learned helpless rats in the forced swim test. Synapse 2004;52:73–5. http://dx.doi.org/10.1002/syn.10308.
Albinsson A, Björk A, Svartengren J, Klint T, Andersson G. Preclinical pharmacology of FG5893: a potential anxiolytic drug with high affinity for both 5-HT1A and 5-HT2A receptors. Eur J Pharmacol 1994;261:285–94.
Marek GJ, Carpenter LL, McDougle CJ, Price LH. Synergistic action of 5-HT2A antagonists and selective serotonin reuptake inhibitors in neuropsychiatric disorders. Neuropsychopharmacology 2003;28:402–12. http://dx.doi.org/10.1038/sj.npp.1300057.
Pehek EA, Nocjar C, Roth BL, Byrd TA, Mabrouk OS. Evidence for the preferential involvement of 5-HT2A serotonin receptors in stress- and drug-induced dopamine release in the rat medial prefrontal cortex. Neuropsychopharmacology 2006;31:265–77. http://dx.doi.org/10.1038/sj.npp.1300819.
Scruggs JL, Schmidt D, Deutch AY. The hallucinogen 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) increases cortical extracellular glutamate levels in rats. Neurosci Lett 2003;346:137–40.
Popa D, Léna C, Fabre V, Prenat C, Gingrich J, Escourrou P, et al. Contribution of 5-HT2 receptor subtypes to sleep-wakefulness and respiratory control, and functional adaptations in knock-out mice lacking 5-HT2Areceptors. J Neurosci 2005;25:11231–38. http://dx.doi.org/10.1523/JNEUROSCI.1724-05.2005.
Adrien J. Implication of serotonin in the control of vigilance states as revealed by knockout-mouse studies. J Soc Biol 2004;198:30–6.
Halberstadt AL, van der Heijden I, Ruderman MA, Risbrough VB, Gingrich JA, Geyer MA, et al. 5-HT(2A) and 5-HT(2C) receptors exert opposing effects on locomotor activity in mice. Neuropsychopharmacology 2009;34:1958–67. http://dx.doi.org/10.1038/npp.2009.29.
Osterlund MK, Overstreet DH, Hurd YL. The Flinders sensitive line rats, a genetic model of depression, show abnormal serotonin receptor mRNA expression in the brain that is reversed by 17beta-estradiol. Brain Res Mol Brain Res 1999;74:158–66.
Rosenzweig-Lipson S, Sabb A, Stack G, Mitchell P, Lucki I, Malberg JE, et al. Antidepressant-like effects of the novel, selective, 5-HT2C receptor agonist WAY-163909 in rodents. Psychopharmacology (Berl) 2007;192:159–70. http://dx.doi.org/10.1007/s00213-007-0710-6.
Dremencov E, Newman ME, Kinor N, Blatman-Jan G, Schindler CJ, Overstreet DH, et al. Hyperfunctionality of serotonin-2C receptor-mediated inhibition of accumbal dopamine release in an animal model of depression is reversed by antidepressant treatment. Neuropharmacology 2005;48:34–42. http://dx.doi.org/10.1016/j.neuropharm.2004.09.013.
Oba A, Nakagawasai O, Onogi H, Nemoto W, Yaoita F, Arai Y, et al. Chronic fluvoxamine treatment changes 5-HT(2A/2C) receptor-mediated behavior in olfactory bulbectomized mice. Life Sci 2013;92:119–24. http://dx.doi.org/10.1016/j.lfs.2012.11.005.
Calcagno E, Guzzetti S, Canetta A, Fracasso C, Caccia S, Cervo L, et al. Enhancement of cortical extracellular 5-HT by 5-HT1A and 5-HT2C receptor blockade restores the antidepressant-like effect of citalopram in non-responder mice. Int J Neuropsychopharmacol 2009;12:793–803. http://dx.doi.org/10.1017/S1461145708009760.
Lucas G, Rymar VV, Du J, Mnie-Filali O, Bisgaard C, Manta S, et al. Serotonin(4) (5-HT(4)) receptor agonists are putative antidepressants with a rapid onset of action. Neuron 2007;55:712–25. http://dx.doi.org/10.1016/j.neuron.2007.07.041.
Meneses A. Stimulation of 5-HT1A, 5-HT1B, 5-HT2A/2C, 5-HT3 and 5-HT4 receptors or 5-HT uptake inhibition: short- and long-term memory. Behav Brain Res 2007;184:81–90. http://dx.doi.org/10.1016/j.bbr.2007.06.026.
Conductier G, Dusticier N, Lucas G, Côté F, Debonnel G, Daszuta A, et al. Adaptive changes in serotonin neurons of the raphe nuclei in 5-HT(4) receptor knock-out mouse. Eur J Neurosci 2006;24:1053–62. http://dx.doi.org/10.1111/j.1460-9568.2006.04943.x.
Gardier AM, Guiard BP, Guilloux J-P, Repérant C, Coudoré F, David DJ. Interest of using genetically manipulated mice as models of depression to evaluate antidepressant drugs activity: a review. Fundam Clin Pharmacol 2009;23:23–42. http://dx.doi.org/10.1111/j.1472-8206.2008.00640.x.
Licht CL, Marcussen AB, Wegener G, Overstreet DH, Aznar S, Knudsen GM. The brain 5-HT4 receptor binding is down-regulated in the Flinders sensitive line depression model and in response to paroxetine administration. J Neurochem 2009;109:1363–74. http://dx.doi.org/10.1111/j.1471-4159.2009.06050.x.
Licht CL, Kirkegaard L, Zueger M, Chourbaji S, Gass P, Aznar S, et al. Changes in 5-HT4 receptor and 5-HT transporter binding in olfactory bulbectomized and glucocorticoid receptor heterozygous mice. Neurochem Int 2010;56:603–10. http://dx.doi.org/10.1016/j.neuint.2010.01.003.
Tecott LH, Chu HM, Brennan TJ. Neurobehavioral analysis of 5-HT6 receptor null mutant mice. In: 4th IUPHAR (International Union Pharmacol.) Satell. Meet. Serotonin; 1998.
Wesolowska A, Nikiforuk A. Effects of the brain-penetrant and selective 5-HT6 receptor antagonist SB-399885 in animal models of anxiety and depression. Neuropharmacology 2007;52:1274–83. http://dx.doi.org/10.1016/j.neuropharm.2007.01.007.
Jastrzębska-Więsek M, Siwek A, Partyka A, Szewczyk B, Sowa-Kućma M, Wasik A, et al. Antidepressant-like activity of EMD 386088, a 5-HT6 receptor partial agonist, following systemic acute and chronic administration to rats. Naunyn Schmiedebergs Arch Pharmacol 2015. http://dx.doi.org/10.1007/s00210-015-1141-2.
Wesołowska A, Nikiforuk A, Stachowicz K. Anxiolytic-like and antidepressant-like effects produced by the selective 5-HT6 receptor antagonist SB-258585 after intrahippocampal administration to rats. Behav Pharmacol 2007;18:439–46. http://dx.doi.org/10.1097/FBP.0b013e3282d28f9c.
Guscott M, Bristow LJ, Hadingham K, Rosahl TW, Beer MS, Stanton JA, et al. Genetic knockout and pharmacological blockade studies of the 5-HT7 receptor suggest therapeutic potential in depression. Neuropharmacology 2005;48:492–502. http://dx.doi.org/10.1016/j.neuropharm.2004.11.015.
Nakagawa Y, Ishima T, Takashima T. The 5-HT3 receptor agonist attenuates the action of antidepressants in the forced swim test in rats. Brain Res 1998;786:189–93.
Bhatt S, Mahesh R, **dal A, Devadoss T. Neuropharmacological effect of novel 5-HT3 receptor antagonist, N-n-propyl-3-ethoxyquinoxaline-2-carboxamide (6n) on chronic unpredictable mild stress-induced molecular and cellular response: behavioural and biochemical evidences. Pharmacol Rep 2014;66:804–10. http://dx.doi.org/10.1016/j.pharep.2014.05.002.
Bhatt S, Mahesh R, **dal A, Devadoss T. Protective effects of a novel 5-HT3 receptor antagonist, N-n-butyl-3-methoxy quinoxaline-2-carboxamide (6o) against chronic unpredictable mild stress-induced behavioral changes and biochemical alterations. Pharmacol Biochem Behav 2014;122:234–9. http://dx.doi.org/10.1016/j.pbb.2014.03.029.
Bhatt S, Radhakrishnan M, **dal A, Devadoss T, Dhar AK. Neuropharmacological evaluation of a novel 5-HT3 receptor antagonist (6g) on chronic unpredictable mild stress-induced changes in behavioural and brain oxidative stress parameters in mice. Indian J Pharmacol 2014;46:191–6. http://dx.doi.org/10.4103/0253-7613.129316.
Bhatnagar S, Nowak N, Babich L, Bok L. Deletion of the 5-HT3 receptor differentially affects behavior of males and females in the Porsolt forced swim and defensive withdrawal tests. Behav Brain Res 2004;153:527–35. http://dx.doi.org/10.1016/j.bbr.2004.01.018.
Elhwuegi AS. Central monoamines and their role in major depression. Prog Neuropsychopharmacol Biol Psychiatry 2004;28:435–51. http://dx.doi.org/10.1016/j.pnpbp.2003.11.018.
Christopher J, Mathias SRB. Autonomic failure a textbook of clinical disorders of the autonomic nervous system. Oxford University Press; 2013.
Cottingham C, Wang Q. α2 adrenergic receptor dysregulation in depressive disorders: implications for the neurobiology of depression and antidepressant therapy. Neurosci Biobehav Rev 2012;36:2214–25. http://dx.doi.org/10.1016/j.neubiorev.2012.07.011.
Hieble JP, Bylund DB, Clarke DE, Eikenburg DC, Langer SZ, Lefkowitz RJ, et al. International Union of Pharmacology, X. Recommendation for nomenclature of alpha 1-adrenoceptors: consensus update. Pharmacol Rev 1995;47:267–70.
Koshimizu T, Tanoue A, Hirasawa A, Yamauchi J, Tsujimoto G. Recent advances in alpha1-adrenoceptor pharmacology. Pharmacol Ther 2003;98:235–44.
Langer SZ. α2-Adrenoceptors in the treatment of major neuropsychiatric disorders. Trends Pharmacol Sci 2015;36:196–202. http://dx.doi.org/10.1016/j.tips.2015.02.006.
Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci 2009;10:211–23. http://dx.doi.org/10.1038/nrn2573.
Day HE, Campeau S, Watson SJ, Akil H. Expression of alpha(1b) adrenoceptor mRNA in corticotropin-releasing hormone-containing cells of the rat hypothalamus and its regulation by corticosterone. J Neurosci 1999;19:10098–106.
Papay R, Gaivin R, Jha A, McCune DF, McGrath JC, Rodrigo MC, et al. Localization of the mouse alpha1A-adrenergic receptor (AR) in the brain: alpha1AAR is expressed in neurons, GABAergic interneurons, and NG2 oligodendrocyte progenitors. J Comp Neurol 2006;497:209–22. http://dx.doi.org/10.1002/cne.20992.
Wang R, Macmillan LB, Fremeau RT, Magnuson MA, Lindner J, Limbird LE. Expression of alpha 2-adrenergic receptor subtypes in the mouse brain: evaluation of spatial and temporal information imparted by 3 kb of 5′ regulatory sequence for the alpha 2A AR-receptor gene in transgenic animals. Neuroscience 1996;74:199–218.
Brosda J, Jantschak F, Pertz HH. α2-Adrenoceptors are targets for antipsychotic drugs. Psychopharmacology (Berl) 2014;231:801–12. http://dx.doi.org/10.1007/s00213-014-3459-8.
Nicholas AP, Pieribone VA, Hökfelt T. Cellular localization of messenger RNA for beta-1 and beta-2 adrenergic receptors in rat brain: an in situ hybridization study. Neuroscience 1993;56:1023–39.
Rodriguez M, Carillon C, Coquerel A, Le Fur G, Ferrara P, Caput D, et al. Evidence for the presence of beta 3-adrenergic receptor mRNA in the human brain. Brain Res Mol Brain Res 1995;29:369–75.
Ordway GA, Klimek V. Noradrenergic pathology in psychiatric disorders: postmortem studies. CNS Spectr 2001;6:697–703.
Arango V, Ernsberger P, Sved AF, Mann JJ. Quantitative autoradiography of alpha 1- and alpha 2-adrenergic receptors in the cerebral cortex of controls and suicide victims. Brain Res 1993;630:271–82.
Meana JJ, García-Sevilla JA. Increased alpha 2-adrenoceptor density in the frontal cortex of depressed suicide victims. J Neural Transm 1987;70:377–81.
Meana JJ, Barturen F, García-Sevilla JA. Alpha 2-adrenoceptors in the brain of suicide victims: increased receptor density associated with major depression. Biol Psychiatry 1992;31:471–90.
De Paermentier F, Cheetham SC, Crompton MR, Katona CL, Horton RW. Brain beta-adrenoceptor binding sites in antidepressant-free depressed suicide victims. Brain Res 1990;525:71–7.
Rivero G, Gabilondo AM, García-Sevilla JA, La Harpe R, Callado LF, Increased Meana JJ. α2- and β1-adrenoceptor densities in postmortem brain of subjects with depression: differential effect of antidepressant treatment. J Affect Disord 2014;167:343–50. http://dx.doi.org/10.1016/j.jad.2014.06.016.
Docherty JR. Subtypes of functional alpha1-adrenoceptor. Cell Mol Life Sci 2010;67:405–17. http://dx.doi.org/10.1007/s00018-009-0174-4.
Cunha MP, Pazini FL, Oliveira Á, Bettio LEB, Rosa JM, Machado DG, et al. The activation of α1-adrenoceptors is implicated in the antidepressant-like effect of creatine in the tail suspension test. Prog Neuropsychopharmacol Biol Psychiatry 2013;44:39–50. http://dx.doi.org/10.1016/j.pnpbp.2013.01.014.
Citó MCO, Silva MIG, Santos LKX, Fernandes ML, Melo FHC, Aguiar JAC, et al. Antidepressant-like effect of Hoodia gordonii in a forced swimming test in mice: evidence for involvement of the monoaminergic system. Braz J Med Biol Res 2015;48:57–64.
Bevilaqua F, Mocelin R, Grimm C, da Silva Junior NS, Buzetto TLB, Conterato GMM, et al. Involvement of the catecholaminergic system on the antidepressant-like effects of Alpinia zerumbet in mice. Pharm Biol 2015;1–6. http://dx.doi.org/10.3109/13880209.2015.1025287.
Martinez DM, Barcellos A, Casaril AM, Savegnago L, Lernardão EJ. Antidepressant-like activity of dehydrozingerone: involvement of the serotonergic and noradrenergic systems. Pharmacol Biochem Behav 2014;127:111–7. http://dx.doi.org/10.1016/j.pbb.2014.10.010.
Piotrowska A, Siwek A, Wolak M, Pochwat B, Szewczyk B, Opoka W, et al. Involvement of the monoaminergic system in the antidepressant-like activity of chromium chloride in the forced swim test. J Physiol Pharmacol 2013;64:493–8.
De Sousa FCF, Oliveira ICM, Silva MIG, de Melo CTV, Santiago VR, de Castro Chaves R, et al. Involvement of monoaminergic system in the antidepressant-like effect of riparin I from Aniba riparia (Nees) Mez (Lauraceae) in mice. Fundam Clin Pharmacol 2014;28:95–103. http://dx.doi.org/10.1111/j.1472-8206.2012.01069.x.
Girish C, Raj V, Arya J, Balakrishnan S. Evidence for the involvement of the monoaminergic system, but not the opioid system in the antidepressant-like activity of ellagic acid in mice. Eur J Pharmacol 2012;682:118–25. http://dx.doi.org/10.1016/j.ejphar.2012.02.034.
Doze VA, Handel EM, Jensen KA, Darsie B, Luger EJ, Haselton JR, et al. alpha(1A)- and alpha(1B)-adrenergic receptors differentially modulate anti-depressant-like behavior in the mouse. Brain Res 2009;1285:148–57. http://dx.doi.org/10.1016/j.brainres.2009.06.035.
Ribeiro CAS, Pupo AS. Involvement of α1B-adrenoceptors in the anti-immobility effect of imipramine in the tail suspension test. Eur J Pharmacol 2015;750:39–42. http://dx.doi.org/10.1016/j.ejphar.2015.01.010.
Hein L, Altman JD, Kobilka BK. Two functionally distinct alpha2-adrenergic receptors regulate sympathetic neurotransmission. Nature 1999;402:181–4. http://dx.doi.org/10.1038/46040.
Gilsbach R, Hein L. Are the pharmacology and physiology of α2 adrenoceptors determined by α2-heteroreceptors and autoreceptors respectively? Br J Pharmacol 2012;165:90–102. http://dx.doi.org/10.1111/j.1476-5381.2011.01533.x.
De Paermentier F, Mauger JM, Lowther S, Crompton MR, Katona CL, Horton RW. Brain alpha-adrenoceptors in depressed suicides. Brain Res 1997;757:60–8.
Pinder RM, Van Delft AM. The potential therapeutic role of the enantiomers and metabolites of mianserin. Br J Clin Pharmacol 1983;15(Suppl. 2):269S–76S.
Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev 2001;7:249–64.
Vega-Rivera NM, López-Rubalcava C, Estrada-Camarena E. The antidepressant-like effect of ethynyl estradiol is mediated by both serotonergic and noradrenergic systems in the forced swimming test. Neuroscience 2013;250:102–11. http://dx.doi.org/10.1016/j.neuroscience.2013.06.058.
Ishola IO, Ochieng CO, Olayemi SO, Jimoh MO, Lawal SM. Potential of novel phytoecdysteroids isolated from Vitex doniana in the treatment depression: involvement of monoaminergic systems. Pharmacol Biochem Behav 2014;127:90–100. http://dx.doi.org/10.1016/j.pbb.2014.11.005.
Schramm NL, McDonald MP, Limbird LE. The alpha(2a)-adrenergic receptor plays a protective role in mouse behavioral models of depression and anxiety. J Neurosci 2001;21:4875–82.
Cottingham C, Li X, Wang Q. Noradrenergic antidepressant responses to desipramine in vivo are reciprocally regulated by arrestin3 and spinophilin. Neuropharmacology 2012;62:2354–62. http://dx.doi.org/10.1016/j.neuropharm.2012.02.011.
Cottingham C, Ferryman CJ, Wang Q. α2 adrenergic receptor trafficking as a therapeutic target in antidepressant drug action. Prog Mol Biol Transl Sci 2015;132:207–25. http://dx.doi.org/10.1016/bs.pmbts.2015.03.007.
Lillethorup TP, Iversen P, Wegener G, Doudet DJM, Landau AM. α2-adreno-ceptor binding in Flinders-sensitive line compared with Flinders-resistant line and Sprague-Dawley rats. Acta Neuropsychiatr 2015;1:8. http://dx.doi.org/10.1017/neu.2015.24.
Landau AM, Phan J-A, Iversen P, Lillethorup TP, Simonsen M, Wegener G, et al. Decreased in vivo α2 adrenoceptor binding in the Flinders sensitive line rat model of depression. Neuropharmacology 2015;91:97–102. http://dx.doi.org/10.1016/j.neuropharm.2014.12.025.
Millan MJ. Multi-target strategies for the improved treatment of depressive states: conceptual foundations and neuronal substrates, drug discovery and therapeutic application. Pharmacol Ther 2006;110:135–370. http://dx.doi.org/10.1016/j.pharmthera.2005.11.006.
Gu L, Liu Y-J, Wang Y-B, Yi L-T. Role for monoaminergic systems in the antidepressant-like effect of ethanol extracts from Hemerocallis citrina. J Ethnopharmacol 2012;139:780–7. http://dx.doi.org/10.1016/jjep.2011.11.059.
Zhang H-T, Huang Y, O’Donnell JM. Antagonism of the antidepressant-like effects of clenbuterol by central administration of beta-adrenergic antagonists in rats. Psychopharmacology (Berl) 2003;170:102–7. http://dx.doi.org/10.1007/s00213-003-1512-0.
Overstreet DH, Stemmelin J, Griebel G. Confirmation of antidepressant potential of the selective beta3 adrenoceptor agonist amibegron in an animal model of depression. Pharmacol Biochem Behav 2008;89:623–6. http://dx.doi.org/10.1016/j.pbb.2008.02.020.
Beaulieu J-M, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 2011;63:182–217. http://dx.doi.org/10.1124/pr.110.002642.
Armando I, Konkalmatt P, Felder RA, Jose PA. The renal dopaminergic system: novel diagnostic and therapeutic approaches in hypertension and kidney disease. Transl Res 2015;165:505–11. http://dx.doi.org/10.1016/j.trsl.2014.07.006.
Shulman JM, De Jager PL, Feany MB. Parkinson’s disease: genetics and pathogenesis. Annu Rev Pathol 2011;6:193–222. http://dx.doi.org/10.1146/annurev-pathol-011110-130242.
Goto Y, Grace AA. The dopamine system and the pathophysiology of schizophrenia: a basic science perspective. Int Rev Neurobiol 2007;78:41–68. http://dx.doi.org/10.1016/S0074-7742(06)78002-3.
Schmidt K, Nolte-Zenker B, Patzer J, Bauer M, Schmidt LG, Heinz A. Psychopathological correlates of reduced dopamine receptor sensitivity in depression, schizophrenia, and opiate and alcohol dependence. Pharmacopsychiatry 2001;34:66–72. http://dx.doi.org/10.1055/s-2001-15184.
Delgado PL. Depression: the case for a monoamine deficiency. J Clin Psychiatry 2000;61(Suppl. 6):7–11.
Klimek V, Schenck JE, Han H, Stockmeier CA, Ordway GA. Dopaminergic abnormalities in amygdaloid nuclei in major depression: a postmortem study. Biol Psychiatry 2002;52:740–8.
Bowden C, Theodorou AE, Cheetham SC, Lowther S, Katona CL, Crompton MR, et al. Dopamine D1 and D2 receptor binding sites in brain samples from depressed suicides and controls. Brain Res 1997;752:227–33.
Rocc P, De Leo C, Eva C, Marchiaro L, Milani AM, Musso R, et al. Decrease of the D4 dopamine receptor messenger RNA expression in lymphocytes from patients with major depression. Prog Neuropsychopharmacol Biol Psychiatry 2002;26:1155–60.
Pei L, Li S, Wang M, Diwan M, Anisman H, Fletcher PJ, et al. Uncoupling the dopamine D1–D2 receptor complex exerts antidepressant-like effects. Nat Med 2010;16:1393–5. http://dx.doi.org/10.1038/nm.2263.
Porsolt RD, Bertin A, Blavet N, Deniel M, Jalfre M. Immobility induced by forced swimming in rats: effects of agents which modify central catecholamine and serotonin activity. Eur J Pharmacol 1979;57:201–10.
Evans J, Sun Y, McGregor A, Connor B. Allopregnanolone regulates neurogenesis and depressive/anxiety-like behaviour in a social isolation rodent model of chronic stress. Neuropharmacology 2012;63:1315–26. http://dx.doi.org/10.1016/j.neuropharm.2012.08.012.
Hellweg R, Zueger M, Fink K, Hörtnagl H, Gass P. Olfactory bulbectomy in mice leads to increased BDNF levels and decreased serotonin turnover in depression-related brain areas. Neurobiol Dis 2007;25:1–7. http://dx.doi.org/10.1016/j.nbd.2006.07.017.
D’Aquila PS, Collu M, Pani L, Gessa GL, Serra G. Antidepressant-like effect of selective dopamine D1 receptor agonists in the behavioural despair animal model of depression. Eur J Pharmacol 1994;262:107–11.
Binfaré RW, Mantovani M, Budni J, Santos ARS, Rodrigues ALS. Involvement of dopamine receptors in the antidepressant-like effect of melatonin in the tail suspension test. Eur J Pharmacol 2010;638:78–83. http://dx.doi.org/10.1016/j.ejphar.2010.04.011.
Guzmán-Gutiérrez SL, Bonilla-Jaime H, Gómez-Cansino R, Reyes-Chilpa R. Linalool and β-pinene exert their antidepressant-like activity through the monoaminergic pathway. Life Sci 2015;128:24–9. http://dx.doi.org/10.1016/j.lfs.2015.02.021.
Pesarico AP, Sampaio TB, Stangherlin EC, Mantovani AC, Zeni G, Nogueira CW. The antidepressant-like effect of 7-fluoro-1,3-diphenylisoquinoline-1-amine in the mouse forced swimming test is mediated by serotonergic and dopaminergic systems. Prog Neuropsychopharmacol Biol Psychiatry 2014;54:179–86. http://dx.doi.org/10.1016/j.pnpbp.2014.06.001.
Do Amaral JF, Silva MIG, de Aquino Neto MR, Moura BA, de Carvalho AMR, Vasconcelos PF, et al. Antidepressant-like effect of bis-eugenol in the mice forced swimming test: evidence for the involvement of the monoaminergic system. Fundam Clin Pharmacol 2013;27:471–82. http://dx.doi.org/10.1111/j.1472-8206.2012.01058.x.
Cunha MP, Machado DG, Capra JC, Jacinto J, Bettio LE, Rodrigues ALS. Antidepressant-like effect of creatine in mice involves dopaminergic activation. J Psychopharmacol 2012;26:1489–501. http://dx.doi.org/10.1177/0269881112447989.
Breuer ME, Groenink L, Oosting RS, Buerger E, Korte M, Ferger B, et al. Antidepressant effects of pramipexole, a dopamine D3/D2 receptor agonist, and 7-OH-DPAT, a dopamine D3 receptor agonist, in olfactory bulbectomized rats. Eur J Pharmacol 2009;616:134–40. http://dx.doi.org/10.1016/j.ejphar.2009.06.029.
Li Y, Zhu ZR, Ou BC, Wang YQ, Tan ZB, Deng CM, et al. Dopamine D2/D3 but not dopamine D1 receptors are involved in the rapid antidepressant-like effects of ketamine in the forced swim test. Behav Brain Res 2015;279:100–5. http://dx.doi.org/10.1016/j.bbr.2014.11.016.
Mahmoudi J, Farhoudi M, Talebi M, Sabermarouf B, Sadigh-Eteghad S. Antidepressant-like effect of modafinil in mice: evidence for the involvement of the dopaminergic neurotransmission. Pharmacol Rep 2015;67:478–84. http://dx.doi.org/10.1016/j.pharep.2014.11.005.
Bjørnebekk A, Mathé AA, Brené S. Isolated Flinders sensitive line rats have decreased dopamine D2 receptor mRNA. Neuroreport 2007;18:1039–43. http://dx.doi.org/10.1097/WNR.0b013e3281668bf7.
Moraga-Amaro R, Gonzalez H, Pacheco R, Stehberg J. Dopamine receptor D3 deficiency results in chronic depression and anxiety. Behav Brain Res 2014;274:186–93. http://dx.doi.org/10.1016/j.bbr.2014.07.055.
Leggio GM, Salomone S, Bucolo C, Platania C, Micale V, Caraci F, et al. Dopamine D(3) receptor as a new pharmacological target for the treatment of depression. Eur J Pharmacol 2013;719:25–33. http://dx.doi.org/10.1016/j.ejphar.2013.07.022.
**ng B, Liu P, Jiang W, Liu F, Zhang H, Cao G, et al. Effects of immobilization stress on emotional behaviors in dopamine D3 receptor knockout mice. Behav Brain Res 2013;243:261–6. http://dx.doi.org/10.1016/j.bbr.2013.01.019.
Jutkiewicz EM, Collins GT, Woods JH. Dopamine D2/D3 receptor agonists produce antidepressant-like effects in the rat forced swim test through co-activation of both receptor subtypes. FASEB J 2008;1:904–6.
Chourbaji S, Brandwein C, Vogt MA, Dormann C, Mueller R, Drescher KU, et al. Dopamine receptor 3 (D3) knockout mice show regular emotional behaviour. Pharmacol Res 2008;58:302–7. http://dx.doi.org/10.1016/j.phrs.2008.09.002.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pytka, K., Podkowa, K., Rapacz, A. et al. The role of serotonergic, adrenergic and dopaminergic receptors in antidepressant-like effect. Pharmacol. Rep 68, 263–274 (2016). https://doi.org/10.1016/j.pharep.2015.08.007
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.pharep.2015.08.007