Abstract
Background
The addictive potential of propofol has been scientifically discussed. Drugs’ psychostimulant properties that can be assessed via measurements of locomotor activity are linked to their addictive properties. No studies that have investigated the effects of propofol on locomotor activity have been reported to date. The present study sought to investigate the effects and possible mechanisms of action of propofol on locomotor activity in rats.
Methods
Adult male albino Wistar rats (250–330 g) were used as subjects. The locomotor activities of the rats were recorded for 30 min immediately following intraperitoneal administration of propofol (20 and 40 mg/kg), saline or vehicle (n = 8 for each group). NG-nitro arginine methyl ester (l-NAME, 15–60 mg/kg), a nitric oxide (NO) synthase inhibitor, and haloperidol (0.125–5 mg/kg), a non-specific dopamine receptor antagonist, were also administered to other groups of rats 30 min prior to the propofol (40 mg/kg) injections, and locomotor activity was recorded for 30 min immediately after propofol administration (n = 8 for each group).
Results
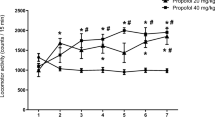
Propofol produced significant increases in the locomotor activities of the rats in the first 5 min of the observation period [F(2,21) = 9.052; p < 0.001]. l-NAME [F(4,35) = 3.112; p = 0.02] but not haloperidol [F(4,35) = 2.440; p = 0.067] pretreatment blocked the propofol-induced locomotor hyperactivity. l-NAME did not cause any significant change in locomotor activity in naïve rats [F(2,21) = 0.569; p = 0.57].
Conclusions
Our results suggest that propofol might cause a short-term induction of locomotor activity in rats and that this effect might be related to nitrergic but not dopaminergic mechanisms.
Similar content being viewed by others
References
Baker MT. The anticonvulsant effects of propofol and a propofol analog, 2,6-diisopropyl-4-(1-hydroxy-2,2,2-trifluoroethyl)phenol, in a 6 Hz partial seizure model. Anesth Analg 2011;112(2):340–4.
Fulton B, Propofol Sorkin EM. An overview of its pharmacology and a review of its clinical efficacy in intensive care sedation. Drugs 1995;50(4):636–57.
Follette JW, Farley WJ. Anesthesiologist addicted to propofol. Anesthesiology 1992;77(4):817–8.
Gepts E, Claeys MA, Camu F, Smekens L. Infusion of propofol (Diprivan) as sedative technique for colonoscopies. Postgrad Med J 1985;61(Suppl. 3):120–6.
Grant IS, Mackenzie N. Recovery following propofol (Diprivan) anaesthesia – a review of three different anaesthetic techniques. Postgrad Med J 1985;61(Suppl. 3):133–7.
Borgeat A, Wilder-Smith OH, Suter PM. The nonhypnotic therapeutic applications of propofol. Anesthesiology 1994;80(3):642–56.
Monroe T, Hamza H, Stocks G, Scimeca PD, Cowan R. The misuse and abuse of propofol. Subst Use Missue 2011;46(9):1199–205.
Bonnet U, Harkener J, Scherbaum N. A case report of propofol dependence in a physician. J Psychoactive Drugs 2008;40(2):215–7.
Bonnet U, Scherbaum N. Craving dominates propofol addiction of an affected physician. J Psychoactive Drugs 2012;44(2):186–90.
Earley PH, Finver T. Addiction to propofol: a study of 22 treatment cases. J Addict Med 2013;7(3):169–76.
Fritz GA, Niemczyk WE. Propofol dependency in a lay person. Anesthesiology 2002;96(2):505–6.
Roh S, Park JM, Kim DJ. A case of propofol dependence after repeated use for endoscopy. Endoscopy 2011;43(Suppl. 2). UCTN:E362.
Wilson C, Canning P, Caravati EM. The abuse potential of propofol. Clin Toxicol 2010;48(3):165–70.
Pain L, Oberling P, Sandner G, Di Scala G. Effect of propofol on affective state as assessed by place conditioning paradigm in rats. Anesthesiology 1996;85(1): 121–8.
Pain L, Oberling P, Sandner G, Di Scala G. Effect of midazolam on propofol-induced positive affective state assessed by place conditioning in rats. Anesthesiology 1997;87(4):935–43.
Barros-Pereira M, Pego JM, Sousa N. Anxiolytic effects of propofol with nonsedative doses. Eur J Anaestiol 2005;22:138.
Kurt M, Bilge SS, Kukula O, Celik S, Kesim Y. Anxiolytic-like profile of propofol, a general anesthetic, in the plus-maze test in mice. Pol J Pharmacol 2003;55(6):973–7.
Schuckit MA. Drug and alcohol abuse: a clinical guide to diagnosis and treatment. 5th ed. New York: Kluwer Academic/Plenum Publishers; 2000. p. 28–53.
Weerts EM, Ator NA, Griffiths RR. Comparison of the intravenous reinforcing effects of propofol and metohexital in baboons. Drug Alcohol Depend 1999; 57(1):51–60.
Le Sage MG, Stafford D, Glowa JR. Abuse liability of the anaesthetic propofol: self-administration of propofol in rats under fixed-ratio schedules of drug delivery. Psychopharmacology 2000;153(1):148–54.
Blokhina EA, Dravolina OA, Bespalov AY, Balster RL, Zvartu EE. Intravenous self-administration of abused solvents and anesthetics in mice. Eur J Pharmacol 2004;485(1-3):211–8.
Lian Q, Wang B, Zhou W, ** S, Xu L, Huang Q, et al. Self-administration of propofol is mediated by dopamine D1 receptors in nucleus accumbens in rats. Neuroscience 2013;231(February):373–83.
Li K-Y, **ao C, **ong M, Delphin E, Ye J-H. Nanomolar propofol stimulates glutamate transmission to dopamine neurons: a possible mechanism of abuse potential. J Pharmacol Exp Ther 2008;325(1):165–74.
Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev 1987;94(4):469–92.
Uzbay IT, Wallis CJ, Lal H, Forster MJ. Effects of NMDA receptor blockers on cocaine-stimulated locomotor activity in mice. Behav Brain Res 2000;108(1): 57–61.
Celik T, Zagli U, Kayir H, Uzbay IT. Nitric oxide synthase inhibition blocks amphetamine-induced locomotor activity in mice. Drug Alcohol Depend 1999;56(2):109–13.
Kayir H, Uzbay IT. Evidence for the role of nitric oxide in caffeine-induced locomotor activity in mice. Psychopharmacology 2004;172(1):11–5.
Uzbay T, Kose A, Kayir H, Ulusoy G, Celik T. Sex-related effects of agmatine on caffeine-induced locomotor activity in Swiss Webster mice. Eur J Pharmacol 2010;630(1–3):69–73.
Frye GD, Breese GR. An evaluation of the locomotor stimulating action of ethanol in rats and mice. Psychopharmacology 1981;75(4):372–9.
Uzbay IT, Kayir H. Bromocriptine and quinpirole but not 7-OH-DPAT and SKF 38393 potentiate the inhibitory effect of L-NAME on ethanol-induced locomotor activity in mice. Naunyn-Schmi Arch Pharmacol 2003;367(4):414–21.
Ulusu U, Uzbay IT, Kayir H, Alici T, Karakas S. Evidence for the role of nitric oxide in nicotine-induced locomotor sensitization in mice. Psychopharmacology 2005;178(4):500–4.
Wright JM, Dobosiewicz MR, Clarke PB. The role of dopaminergic transmission through D1-like and D2-like receptors in amphetamine-induced rat ultrasonic vocalizations. Psychopharmacology 2013;225(4):853–68.
Ozden O, Kayir H, Ozturk Y, Uzbay T. Agmatine blocks ethanol-induced locomotor hyperactivity in male mice. Eur J Pharmacol 2011;659(1):26–9.
Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci 2005;8(11):1445–9.
Pain L, Gobaille S, Schleef C, Aunis D, Oberling P. In vivo dopamine measurements in the nucleus accumbens after nonanesthetic and anesthetic doses of propofol in rats. Anest Analg 2002;95(4):915–9.
Amos S, Chindo BA, Abbah J, Vongtau HO, Edmond I,Binda L,et al. Postsynaptic dopamine D2-mediated behavioural effects of high acute doses of artemisinin in rodents. Brain Res Bull 2003;62(3):255–60.
Kayir H, Ceyhan M, Yavuz O, Uzbay IT. Lack of effect of Nomega-nitro-L-arginine methyl ester on bromocriptine-induced locomotor sensitization in mice. Synapse 2007;61(10):869–75.
Grasshoff C, Herrera-Marschitz M, Goiny M, Kretschmer BD. Modulation of ventral pallidal dopamine and glutamate release by the intravenous anesthetic propofol studied by in vivo microdialysis. Amino Acids 2005; 28(2):145–8.
Uzbay IT, Oglesby MW. Nitric oxide and substance dependence. Neurosci Biobehav Rev 2001;25(1):43–52.
Tonner PH, Scholz J, Lamberz L, Schlamp N, Schulte am Esch J. Inhibition of nitric oxide synthase decreases anesthetic requirements of intravenous anesthetics in Xenopus laevis. Anesthesiology 1997;87(6):1479–85.
Itzhak Y, Martin JL. Blockade of alcohol-induced locomotor sensitization and conditioned place preference in DBA mice by 7-nitroindazole. Brain Res 2000;858(2):402–7.
Itzhak Y, Anderson KL. Ethanol-induced behavioral sensitization in adolescent and adult mice: role of the nNOS gene. Alcohol Clin Exp Res 2008;32(10): 1839–49.
Itzhak Y, Roger-Sánchez C, Anderson KL. Role of the nNOS gene in ethanol-induced conditioned place preference in mice. Alcohol 2009;43(4):285–91.
Garthwaite J, Garthwaite G, Palmer RM, Moncada S. NMDA receptor activation induces nitric oxide synthesis from arginine in rat brain slices. Eur J Pharmacol 1989;172(4–5):413–6.
Garthwaite J. Glutamate, nitric oxide and cell-cell signaling in the nervous system. Trends Neurosci 1991;14(2):60–7.
Zhu H, Cottrell JE, Kass IS. The effect of thiopental and propofol on NMDA-and AMPA-mediated glutamate excitotoxicity. Anesthesiology 1997;87(4): 944–51.
Kosowski AR, Cebers G, Cebere A, Swanhagen AC, Liljequist S. Nicotine-induced dopamine release in the nucleus accumbens is inhibited by the novel AMPA antagonist ZK200775 and the NMDA antagonist CGP39551. Psychopharmacology 2004;175(1):114–23.
Kozinn J, Mao L, Yang L, Fibuch EE, Wang JQ. Inhibition of glutamatergic activation of extracellular signal-regulated protein kinases in hippocampal neurons by the intravenous anesthetic propofol. Anesthesiology 2006;105(6): 1182–91.
Ito H, Watanabe Y, Isshiki A, Uchino H. Neuroprotective properties of propofol and midazolam, but not pentobarbital, on neuronal damage induced by forebrain ischemia, based on the GABAA receptors. Acta Anaesthesiol Scand 1999;43(2):153–62.
Schwieler L, Delbro D, Engberg G, Erhardt S. The anaesthetic agent propofol interacts with GABA(B)-receptors: an electrophysiological study in rat. Life Sci 2003;72(24):2793–801.
Nutt D, Lingford-Hughes A. Addiction: the clinical interface. Br J Pharmacol 2008;154(2):397–405.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tezcan, A.H., Özçetin, A., Özlü, O. et al. Locomotor stimulation by acute propofol administration in rats: Role of the nitrergic system. Pharmacol. Rep 67, 980–985 (2015). https://doi.org/10.1016/j.pharep.2015.03.003
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.pharep.2015.03.003