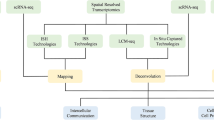
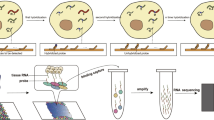
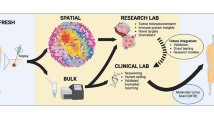
Abstract
Tumor research is a fundamental focus of medical science, yet the intrinsic heterogeneity and complexity of tumors present challenges in understanding their biological mechanisms of initiation, progression, and metastasis. Recent advancements in single-cell transcriptomic sequencing have revolutionized the way researchers explore tumor biology by providing unprecedented resolution. However, a key limitation of single-cell sequencing is the loss of spatial information during single-cell preparation. Spatial transcriptomics (ST) emerges as a cutting-edge technology in tumor research that preserves the spatial information of RNA transcripts, thereby facilitating a deeper understanding of the tumor heterogeneity, the intricate interplay between tumor cells and the tumor microenvironment. This review systematically introduces ST technologies and summarizes their latest applications in tumor research. Furthermore, we provide a thorough overview of the bioinformatics analysis workflow for ST data and offer an online tutorial (https://github.com/SiyuanHuang1/ST_Analysis_Handbook). Lastly, we discuss the potential future directions of ST. We believe that ST will become a powerful tool in unraveling tumor biology and offer new insights for effective treatment and precision medicine in oncology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Despite ongoing advancements in the field of oncology, cancer remains a significant threat to human health, primarily due to its inherent complexity. There is a broad consensus that cancer cells are not solitary entities; rather, they engage in intricate interactions with adjacent immune and stromal cells. This complex interplay contributes to the formation of an advanced tumor microenvironment that is fundamentally involved in both the onset and progression of cancer [1]. The advent of molecular targeted therapy and immunotherapy has brought hope for improved cancer treatment. However, the heterogeneous nature of tumors results in only a minority of patients responding effectively to these treatments [2]. Consequently, there is an urgent need for cutting-edge research techniques and innovative approaches in current cancer-related studies.
The spatial architecture of a tumor plays a critical role in its development and progression. This structural trait is markedly pronounced within certain biological niches, where tumor cells and the encompassing microenvironment engage in dynamic reciprocal interactions. These interactions often foster an environment conducive to immune suppression, thereby facilitating tumor immune evasion and posing a significant hurdle to effective cancer therapy [3]. Furthermore, the spatial structure of tumors not only influences their growth but also drives their metastasis [20]. BaristaSeq, in contrast to other methods that employed sequencing by ligation, made use of improved gap-filling padlock probes and employed Illumina synthesis sequencing, offering improved signal-to-noise ratio detection [21]. This technique was commonly used for sequencing a single barcode per cell [19]. ExSeq combined ISS or FISSEQ with expansion microscopy, allowing targeted or non-targeted detection of RNA [22].
Both ISH and ISS methods utilize pre-designed probes and high-resolution microscopy for detection, and enable the analysis of mRNA at subcellular resolution. RCA plays a critical role in enhancing the signal-to-noise ratio, enabling ISS to achieve fewer accumulated errors with fewer hybridization rounds and detect larger tissue areas with lower magnification. However, due to the lower efficiency of RCA amplification and its stronger selectivity [9], ISS can detect fewer genes than ISH. Single-molecule microscopy imaging technology is highly complex, requiring precise instruments and procedures. Additionally, the image data generated using this technology often reach terabytes. The incorporation of multiple imaging cycles and multiple hybridization rounds further consume longer experimental periods and numerous sample handling steps. These factors contribute to the complexity and generally higher experimental costs of ISH and ISS technologies.
Laser capture microdissection-based sequencing methods
Laser capture microdissection (LCM) transfer and tissue analysis were proposed in 1996, initially utilized for polymerase chain reaction amplification and enzyme recovery in specific tissue regions [23]. Subsequently, LCM was combined with comprehensive transcriptome analysis to facilitate the detailed examination of specific cell groups. Although this approach generally required a substantial number of cells, it presents a notable advantage by eliminating the need for tissue dissociation. The Tomo-seq technique was developed to attain spatially resolved, genome-wide expression profiles [24]. By combining LCM with full-length mRNA-sequencing, a robust and highly efficient strategy (LCM-seq) was developed for single-cell transcriptomics. This strategy provided biological insights into the characteristics and functions of similar neuronal populations [25]. Geographical position sequencing (Geo-seq) was a technique that fused laser capture microdissection with single-cell RNA-seq technology to investigate cellular diversity and spatial variability simultaneously [26]. Spatial-histopathological examination-linked epitranscriptomics converged to transcriptomics with sequencing (Select-seq) enabled the acquisition of both transcriptomic and epitranscriptomic data [27].
Several ST technologies employed light-controlled methods to select the region of interest (ROI) instead of involving tissue sectioning. These techniques could be categorized as LCM-like ST technologies. NICHE-seq enabled the isolation and examination of cells from visually chosen specific areas in transgenic mice expressing a photoactivatable green fluorescent protein [28]. This approach was utilized to pinpoint distinct niches for T and B cells within the lymph nodes and spleens of mice following viral infection. ZipSeq used patterned illumination and photocaged oligonucleotides to serially print barcodes onto live cells in intact tissues, allowing real-time and on-the-fly selection of patterns [29]. Digital Spatial Profiling (DSP) was an advanced technique well-suited for formalin-fixed, paraffin-embedded (FFPE) samples, providing detailed spatial analysis of proteins or RNAs [30]. Light-Seq was designed for the in situ spatial labeling of target molecules within specified ROIs, enabling ex situ next-generation sequencing without damaging the sample [31]. Although LCM methods had notable advantages, they typically offered limited spatial resolution and throughput compared to other ST techniques.
ST technology based on spatial barcode
The concept of ST technology based on spatial barcode was first proposed in 2016 [32]. This method identified messenger RNA transcripts by transferring RNA molecules onto a glass slide. The slide was coated with reverse-transcription oligo(dT) primers that contained a unique molecular identifier (UMI) and a spatial barcode, enabling the retrieval of original transcript locations. Although this technique has innovated the approach to transcriptome research, it faced challenges in terms of low RNA capturing efficiency and limited spatial resolution, with a spot diameter of 100 μm and a spot-to-spot distance of 200 μm. To address this limitation, 10 × Genomics reformed and commercialized the method. achieving a higher resolution with a spot diameter of about 55 μm and a spot-to-spot distance of approximately 100 μm. Another approach, Slide-seq, utilized polystyrene beads randomly distributed on a slide, with each bead carrying a unique barcode and attains a spatial resolution of 10 μm [33, 34]. High-definition spatial transcriptomics (HDST) employed a silicon wafer as the base, further improving the spatial resolution to 2 μm [35]. However, the advanced spatial resolution achieved by both Slide-seq and HDST hinges on the random allocation of spatially barcoded beads. Consequently, pinpointing their precise spatial arrangement necessitated labor-intensive imaging-based in situ sequencing techniques. The DBiT-seq (deterministic barcoding in tissue for spatial omics sequencing) method enabled the simultaneous map** of mRNAs and proteins in formaldehyde-fixed tissue sections by combining microfluidic barcoding with next-generation sequencing [36]. Seq-Scope was a two-phase sequencing technology that first created a spatially barcoded oligonucleotide array for mRNA capture and then used Illumina NGS to sequence captured mRNAs, linking each to precise array coordinates for high-resolution spatial transcriptomics.[40].
In recent developments in the field of ST based on spatial barcode, two significant breakthroughs emerged. Russell et al. developed Slide-tags, a novel method enabling single-cell (nuclei) resolution [41]. This technique involved 'tagging' cellular nuclei from tissue sections with spatial barcode oligonucleotides, which were derived from DNA-barcoded beads, under ultraviolet exposure. Slide-tags also offered the advantage of enabling the profiling using existing single-cell methods with the addition of spatial positions. Another significant advancement in ST technology based on spatial barcode was the introduction of the Visium HD spatial gene expression assay by 10 × Genomics. This technique employed a whole transcriptome probe panel and achieves single-cell scale resolution within intact tissue sections. The HD array was composed of approximately 12 million 2 µm × 2 µm spatially-barcoded areas without gaps. This significantly enhances spatial resolution compared to the previous Visium platform.
Spatial barcode-based spatial transcriptomics technologies stand out due to their innovative approach to map** mRNA transcripts by translocating them onto slides with reverse-transcription primers that include UMIs and spatial barcodes. This enables precise map** of each transcript's original location, providing a high-resolution spatial context to transcriptome data. Advancements in this domain, such as the development of Slide-seq and HDST, have dramatically enhanced spatial resolution down to 2 µm, enabling the detailed visualization of transcript distribution at near single-cell resolution. The deterministic nature of DBiT-seq and the single-cell spatial resolution of Slide-tags further underscore the rapid evolution of this field, offering unparalleled insights into the cellular composition of tissues while maintaining the integrity of spatial information. These methods surpass other spatial transcriptomics techniques by allowing for extensive multiplexing and the ability to handle whole transcriptomes, which is a significant leap forward in capturing comprehensive cellular behavior within intact tissue environments. Despite challenges such as lower RNA capture efficiency and the need for complex imaging to pinpoint bead arrangements, the benefits of these techniques, particularly their enhanced resolution and capacity for detailed spatial map**, position them as powerful tools for advancing our understanding of complex biological systems.
Application of ST in cancer research
Understanding the TME and tumor heterogeneity is crucial for effective cancer treatment and prognosis. Traditional single-cell techniques allow researchers to examine and compare genetic and functional features at the single-cell level, unraveling the identities of distinct cell types within complex tissues. This is vital for revealing the intricacies of tumor heterogeneity and the multifaceted complexity of the TME. In order to overcome the limitations of spatial resolution in single-cell RNA sequencing, ST has emerged as a valuable tool for obtaining spatially distributed transcriptomic data from tissue slices. By generating a cell atlas with spatial resolution for solid tumors, this technique facilitates the inference of cell interactions based on co-localization patterns [42], the exploration of tumor infiltration regions and boundaries of the stroma [43], the analysis of spatial immune niches [44], and the identification of specific cell subpopulations with unique distributions within the tissue structure [45]. These insights are crucial for understanding spatial patterns of cell interactions, as well as the distribution of cell types and tumor heterogeneity within the TME. A summary of the applications of ST technology in cancer research is provided in Table 1.
Applications of ST technology in TME
The TME is primarily composed of a diverse range of cell types, including malignant cells, immune cells, stromal cells, neurons, smooth muscle cells, lymphatic vessels, and blood vessels. These cell types can be further classified into various subtypes based on their specific gene expression patterns. Within the TME, these diverse cell types interact spatially, occupying ecological niches to form a complex ecosystem [87]. ST has emerged as a powerful technique for elucidating the ecological structure of the TME by revealing cell types, cell states, and the interactions between different cell types.
ST elucidates the complex interactions within the immune microenvironment by revealing cell-type-specific interactions and structures that are critical for the immune response to cancer. For instance, in a study on HER2-positive breast cancer (HER2 + BC), Andersson et al. explored the spatial gene expression patterns and discovered specific interactions between macrophages and T cell subtypes during the type I interferon response. Furthermore, they identified co-localization between B cells and T cells, unveiling tertiary lymphoid structures (TLS) in a spatial context [46]. Liu et al. combined ST with single-cell RNA sequencing and multi-immunofluorescence to uncover the tumor immune barrier (TIB) structure—a spatial niche composed of SPP + macrophages and cancer-associated fibroblasts (CAFs) located near the tumor boundary. The study demonstrated that the impact of spatial structures on the immunotherapeutic efficacy in hepatocellular carcinoma (HCC) patients receiving anti-PD-1 treatment [53]. Spatial organizational information provided by ST has assisted researchers to propose TLS-50 features for precise spatial localization of TLS, highlighting their unique composition determined by their proximity to tumor cells [51].
The application of ST in cancer research has provided insights into the dynamic remodeling of the TME in response to cancer progression and metastasis, highlighting the evolving landscape of cellular populations and the formation of specialized niches. Hwang et al. conducted single-nucleus RNA sequencing and Digital Spatial Profiling (DSP) analysis on 43 primary pancreatic ductal adenocarcinoma (PDAC) tumor specimens. This approach enabled the construction of a high-resolution spatial map of cell community distribution within the PDAC microenvironment. Through their analysis, they identified expression programs prevalent in both PDAC malignant cells and fibroblasts, unveiling three multicellular communities comprising various cell subtypes within the TME [52]. Qi et al. explored the spatial landscape of primary and metastatic tumors in non-small cell lung cancer (NSCLC) brain metastases (BrMs), and revealed extensive remodeling of the TME in the brain, leading to the formation of an immunosuppressive and fibrotic niche for BrMs [49]. In another study of invasive TME in lung adenocarcinoma (LUAD), Zhu et al. conducted an integrated analysis using single-cell RNA-sequencing (scRNA-seq) and ST to characterize the cellular atlas of LUAD invasion trajectories. They observed a continuous increase in the UBE2C + cancer cell subpopulation during LUAD invasion, while mast cells, monocytes, and lymphatic endothelial cells decreased [92]. This probabilistic model provides improved estimates of UMI counts for every gene at each spot by removing contamination from spot swap**. Furthermore, SpotClean has demonstrated significant improvements in marker gene identification and spatial domain detection.
The shallow depth of ST sequencing is associated with lower capture efficiency, consequently leading to a higher rate of dropouts. Recently, some computational tools have been developed to impute the missing expression levels due to dropouts by aggregating the expression data of the spatially neighbor spots. Furthermore, some of these methods consider the similarity of histological structures observed in histology images when selecting the spatially neighbor spots. One such method, Sprod, employs latent graph learning techniques to integrate gene expression data and imaging data, allowing for the precise imputation and denoising of ST gene expression [108], involves spatial smoothing of gene expression based on morphological similarity and spatial location. Subsequently, it employs a standard Louvain clustering procedure to detect spatial domains. The second category of methods is developed based on the standard Hidden Markov Random Field (HMRF) framework, allowing latent states representing domain categories to be spatially continuous. Representative tools include BayesSpace [97], Giotto [109], SC-MEB [110], DR-SC [111] and BASS [112]. Taking Giotto as an example, it first selects spatially differentially expressed genes and then utilizes HMRF clustering to detect spatial domains. The third category employs graph neural networks (GNNs) to process graphs derived from spatial locations, expression data, and potentially histology image information. This approach enhances spot embeddings through learning the complex relationships between spots, ultimately leading to improved detection of spatial domains and gene expression patterns. Representative algorithms include SpaGCN [113], STAGATE [114], CCST [115], and GraphST [116]. For example, GraphST integrates graph neural networks with self-supervised contrastive learning to improve the representations of spots. It optimizes the embedding distances among spatially neighboring or distant spots to acquire informative and discriminative spot representations, finally enabling spatially informed clustering, integration, and deconvolution.
Deconvolution
Deconvolution, a widely employed method in bulk RNA-seq to differentiate mixed expression signals into identifiable cell types, becomes exceedingly useful in the realm of spatial transcriptomics, such as with Visium platforms, where each spot aggregates transcripts from several cells. This method typically uses a cell-type-annotated single-cell sequencing data as a reference, facilitating accurate estimation of cell type proportions within each spot, thereby illuminating the intricacies of tissue microenvironments and the underlying cellular architecture. Several tools in this field have been developed to perform deconvolution effectively, such as SPOTlight [117], SpatialDWLS [118], DSTG [130], cell2location [119], CARD [120], RCTD [123], destVI [121] and STRIDE [122]. SPOTlight utilizes seeded non-negative matrix factorization (NMF) regression to deconvolute the ST information with scRNA-seq data. DSTG generates pseudo-ST data by simulating cell mixtures from scRNA-seq data and utilizes graph-based convolutional networks for deconvolution. Cell2location, a Bayesian model, demonstrates the ability to resolve fine-grained cell types in ST data and integrate single-cell and ST with high sensitivity and resolution. To assess and compare the performance of these algorithms, benchmarking studies have been conducted, providing valuable insights for selecting appropriate methods for cell type deconvolution of spots [143, 144].
Another category of deconvolution methods, known as reference-free deconvolution methods, does not require single-cell transcriptomic data as a reference. Representative methods in this category include CARDfree [120], STdeconvolve [124], and SMART [125]. In spatial transcriptomic samples of tumors, the estimation of cancer cell abundance can be particularly challenging due to the heterogeneity of tumor cells. SpaCET [126] addresses this challenge by integrating a gene pattern dictionary of copy number alterations and expression changes to estimate cancer cell abundance. Furthermore, it employs a constrained regression model to determine the proportions of immune and stromal cell lineages. However, these methods have a limitation in that they can only estimate the proportions of cell types in a given spot without providing single-cell level deconvolution.
An alternative approach is to enhance ST data to achieve single-cell resolution. Tools like CellTrek [127] and CytoSPACE [128] assign the most probable spatial location in ST data to each single cell and subsequently estimate the cellular composition within the tissue. SpatialScope [145]. Consequently, it only tests combinations of clusters if they coexist within the same microenvironment.
SpaOTsc uses optimal transport map** between scRNA-seq data and spatial data to construct a spatial metric for cells in scRNA-seq data, and reconstruct space-constrained cell–cell communication networks [134]. The main author of SpaOTsc later proposed COMMOT, which considers the spatial distances between cells and aids in deducing the competitive dynamics between different ligands and receptors [135]. This approach provides a new perspective for examining the spatial interactions between immune cells and tumor cells in the tumor ecosystem.
Alignment and integration of multiple slices
The alignment and integration of multi-slice ST data represents a current challenge in spatial data analysis, which can be broadly categorized into three types: reconstructing three-dimensional (3D) structures, identifying spatial shared domain, and revealing dynamics in biological processes.
For the reconstruction of 3D structures, several methods such as PASTE [138], PASTE2 [139], SLAT [86]. However, some ST technologies, such as Slide-seq and HDST, are not yet applicable to FFPE samples [148]. In the future, spatial omics technologies adapted for FFPE samples will accelerate research related to tumors, especially in longitudinal studies on tumor progression or disease relapse.
Spatial multi-omics and full-length technology
Following the pioneering development of ST, other spatial omics technologies have increasingly garnered attention. Spatial proteomics technologies have emerged as powerful tools capable of detecting the levels of dozens of proteins in situ. Spatial proteomics primarily fall into two categories: mass spectrometry-based [149,150,151] and antibody-based approaches [152, 153]. Additionally, spatial metabolomics technologies enable non-targeted detection of metabolites and lipids on tissue sections using imaging mass spectrometry [154]. In recent years, several other types of spatial omics technologies have been developed. Spatial ATAC-seq enables in situ sequencing of chromatin to understand chromatin accessibility related to tissue structure and spatial position [86]. This approach may introduce heterogeneity among the sections and pose challenges in data analysis, particularly in aligning multiple sections. Another strategy involves performing multiple omics sequencing on the same section. Compared to the first approach, this method conserves samples and eliminates the necessity for aligning sections. However, it presents greater technical challenges, which is why such technologies are still relatively rare. For instance, spatial CITE-seq [159] and STARmap PLUS [160] add a protein dimension to the capture of spatial transcripts. Slide-TCR-seq enables simultaneous sequencing of TCR and RNA on a single tissue Sect. [161]. In the future, the integration of spatial multi-omics will revolutionize our understanding of how cellular programs are coordinated and the intricate mechanisms underlying tumor biology.
It is worth noting that the current ST approach employed by Visium can only detect information at the 3’ end of transcripts, thus neglecting full-length exploration. This limitation considerably restricts the investigation of immune cell receptor repertoire and alternative splicing events. To improve the accuracy and continuity of sequencing reads, long-read sequencing technologies from Pacific Biosciences (PacBio) [162] and Oxford Nanopore Technologies (ONT) [163] emerge as pivotal tools. Innovative sequencing strategies that integrate long-read sequencing with either bulk or single-cell sequencing [164, 165], along with computational methods [Analysis platforms suitable for clinical practitioners As mentioned previously, the analysis of ST data remains complex due to the diversity of data types, lacking a unified paradigm similar to single-cell transcriptomic analysis. This poses significant challenges for biologists and clinical practitioners. To alleviate this hurdle, several online platforms have emerged to streamline the showcase and exploration of spatial transcriptomic data from various sequencing platforms. Two noteworthy databases, Spatial Omics DataBase (SODB) [174] and Spatial TranscriptOmics DataBase (STOmicsDB) [175] stand out in providing comprehensive resources. In the realm of spatial omics, SODB is a pivotal online platform meticulously designed to provide researchers with an extensive collection of data resources and diverse interactive modules for sophisticated data analysis. Encompassing over 2,400 experiments from more than 25 different spatial omics technologies, SODB maintains data in a standardized format that is compatible with numerous computational tools, ensuring versatility and accessibility. Notably, SODB features a range of interactive modules for data analysis. Another key resource is STOmicsDB, a comprehensive database designed as a central resource for ST research. STOmicsDB contains 218 carefully curated datasets from 17 species, with detailed annotations of cell types, spatial regions, genes, and analyses of cell-to-cell interactions. Its user-friendly interface enables quick visualization of data involving millions of cells. In summary, ST technology presents several areas for improvement and refinement in the future. As these advancements unfold, it is anticipated to emerge as a powerful tool for biologists and clinical scientists, enabling in-depth exploration of the intricate mechanisms underlying tumors.
Conclusions and perspectives
In spite of the clinical implementation and notable successes of targeted and immunotherapy, cancer remains one of the major health challenges faced by humanity. The effectiveness of cancer treatment is primarily hindered by the heterogeneity of tumors and the intricate nature of the TME. Factors such as tumor metastasis, the development of drug resistance, and the presence of an immunosuppressive microenvironment often contribute to treatment failures.
ST, an emerging technology in recent years, offers resolution comparable to single-cell transcriptomics while addressing the lack of spatial context in single-cell sequencing. ST has gained increasing attention from researchers as a powerful tool for studying tumor heterogeneity, the TME, and the spatial structure of tumors, thereby laying an important foundation for research in tumor biology. This review aims to provide a comprehensive overview of the developmental trajectory and recent applications of ST in cancer research. To facilitate comprehension among clinical practitioners and biomedical professionals, a thorough description of the ST data analysis process is included, supplemented by an online guide.
With ST advancing towards enhanced resolution, higher sequencing throughput, multimodality, and reduced costs, its significance in cancer biology research is anticipated to grow substantially. We believe that ST will establish a theoretical foundation for personalized precision medicine, making significant contributions to the development of targeted and individualized therapeutic approaches in the field of oncology.
Availability of data and material
Not applicable.
References
Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019;79(18):4557–66.
Lawson DA, Kessenbrock K, Davis RT, Pervolarakis N, Werb Z. Tumour heterogeneity and metastasis at single-cell resolution. Nat Cell Biol. 2018;20(12):1349–60.
Kim SK, Cho SW. The evasion mechanisms of cancer immunity and drug intervention in the tumor microenvironment. Front Pharmacol. 2022;13: 868695.
Fu T, Dai LJ, Wu SY, **ao Y, Ma D, Jiang YZ, et al. Spatial architecture of the immune microenvironment orchestrates tumor immunity and therapeutic response. J Hematol Oncol. 2021;14(1):98.
Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005;437(7057):376–80.
Tang F, Barbacioru C, Wang Y, Nordman E, Lee C, Xu N, et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nat Methods. 2009;6(5):377–82.
Kong D, Huang S, Miao X, Li J, Wu Z, Shi Y, et al. The dynamic cellular landscape of grafts with acute rejection after heart transplantation. J Heart Lung Transplant. 2023;42(2):160–72.
Song G, Shi Y, Meng L, Ma J, Huang S, Zhang J, et al. Single-cell transcriptomic analysis suggests two molecularly subtypes of intrahepatic cholangiocarcinoma. Nat Commun. 2022;13(1):1642.
Moses L, Pachter L. Museum of spatial transcriptomics. Nat Methods. 2022;19(5):534–46.
Gall JG, Pardue ML. Formation and detection of RNA-DNA hybrid molecules in cytological preparations. Proc Natl Acad Sci U S A. 1969;63(2):378–83.
Femino AM, Fay FS, Fogarty K, Singer RH. Visualization of single RNA transcripts in situ. Science. 1998;280(5363):585–90.
Lubeck E, Coskun AF, Zhiyentayev T, Ahmad M, Cai L. Single-cell in situ RNA profiling by sequential hybridization. Nat Methods. 2014;11(4):360–1.
Chen KH, Boettiger AN, Moffitt JR, Wang S, Zhuang X. Spatially resolved, highly multiplexed RNA profiling in single cells. Science. 2015;348(6233):aaa6090.
Eng C-HL, Lawson M, Zhu Q, Dries R, Koulena N, Takei Y, et al. Transcriptome-scale super-resolved imaging in tissues by RNA seqFISH+. Nature. 2019;568(7751):235–9.
**a C, Fan J, Emanuel G, Hao J, Zhuang X. Spatial transcriptome profiling by MERFISH reveals subcellular RNA compartmentalization and cell cycle-dependent gene expression. Proc Natl Acad Sci U S A. 2019;116(39):19490–9.
Park HE, Jo SH, Lee RH, Macks CP, Ku T, Park J, et al. Spatial transcriptomics: technical aspects of recent developments and their applications in neuroscience and cancer research. Adv Sci. 2023;10:2206939.
Ke R, Mignardi M, Pacureanu A, Svedlund J, Botling J, Wahlby C, et al. In situ sequencing for RNA analysis in preserved tissue and cells. Nat Methods. 2013;10(9):857–60.
Lee JH, Daugharthy ER, Scheiman J, Kalhor R, Yang JL, Ferrante TC, et al. Highly multiplexed subcellular RNA sequencing in situ. Science. 2014;343(6177):1360–3.
Bressan D, Battistoni G, Hannon GJ. The dawn of spatial omics. Science. 2023;381(6657):eabq4964.
Wang X, Allen WE, Wright MA, Sylwestrak EL, Samusik N, Vesuna S, et al. Three-dimensional intact-tissue sequencing of single-cell transcriptional states. Science. 2018;361(6400):eaat5691.
Chen X, Sun YC, Church GM, Lee JH, Zador AM. Efficient in situ barcode sequencing using padlock probe-based BaristaSeq. Nucleic Acids Res. 2018;46(4):e22.
Alon S, Goodwin DR, Sinha A, Wassie AT, Chen F, Daugharthy ER, et al. Expansion sequencing: spatially precise in situ transcriptomics in intact biological systems. Science. 2021;371(6528):eaax2656.
Emmert-Buck MR, Bonner RF, Smith PD, Chuaqui RF, Zhuang Z, Goldstein SR, et al. Laser capture microdissection. Science. 1996;274(5289):998–1001.
Junker JP, Noel ES, Guryev V, Peterson KA, Shah G, Huisken J, et al. Genome-wide RNA Tomography in the zebrafish embryo. Cell. 2014;159(3):662–75.
Nichterwitz S, Chen G, Aguila Benitez J, Yilmaz M, Storvall H, Cao M, et al. Laser capture microscopy coupled with Smart-seq2 for precise spatial transcriptomic profiling. Nat Commun. 2016;7:12139.
Chen J, Suo S, Tam PP, Han JJ, Peng G, **g N. Spatial transcriptomic analysis of cryosectioned tissue samples with Geo-seq. Nat Protoc. 2017;12(3):566–80.
Lee AC, Lee Y, Choi A, Lee HB, Shin K, Lee H, et al. Spatial epitranscriptomics reveals A-to-I editome specific to cancer stem cell microniches. Nat Commun. 2022;13(1):2540.
Medaglia C, Giladi A, Stoler-Barak L, De Giovanni M, Salame TM, Biram A, et al. Spatial reconstruction of immune niches by combining photoactivatable reporters and scRNA-seq. Science. 2017;358(6370):1622–6.
Hu KH, Eichorst JP, McGinnis CS, Patterson DM, Chow ED, Kersten K, et al. ZipSeq: barcoding for real-time map** of single cell transcriptomes. Nat Methods. 2020;17(8):833–43.
Merritt CR, Ong GT, Church SE, Barker K, Danaher P, Geiss G, et al. Multiplex digital spatial profiling of proteins and RNA in fixed tissue. Nat Biotechnol. 2020;38(5):586–99.
Kishi JY, Liu N, West ER, Sheng K, Jordanides JJ, Serrata M, et al. Light-Seq: light-directed in situ barcoding of biomolecules in fixed cells and tissues for spatially indexed sequencing. Nat Methods. 2022;19(11):1393–402.
Stahl PL, Salmen F, Vickovic S, Lundmark A, Navarro JF, Magnusson J, et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science. 2016;353(6294):78–82.
Rodriques SG, Stickels RR, Goeva A, Martin CA, Murray E, Vanderburg CR, et al. Slide-seq: A scalable technology for measuring genome-wide expression at high spatial resolution. Science. 2019;363(6434):1463–7.
Stickels RR, Murray E, Kumar P, Li J, Marshall JL, Di Bella DJ, et al. Highly sensitive spatial transcriptomics at near-cellular resolution with Slide-seqV2. Nat Biotechnol. 2021;39(3):313–9.
Vickovic S, Eraslan G, Salmen F, Klughammer J, Stenbeck L, Schapiro D, et al. High-definition spatial transcriptomics for in situ tissue profiling. Nat Methods. 2019;16(10):987–90.
Liu Y, Yang M, Deng Y, Su G, Enninful A, Guo CC, et al. High-spatial-resolution multi-omics sequencing via deterministic barcoding in tissue. Cell. 2020;183(6):1665–81 e18.
Cho CS, ** J, Si Y, Park SR, Hsu JE, Kim M, et al. Microscopic examination of spatial transcriptome using Seq-scope. Cell. 2021;184(13):3559–72 e22.
Srivatsan SR, Regier MC, Barkan E, Franks JM, Packer JS, Grosjean P, et al. Embryo-scale, single-cell spatial transcriptomics. Science. 2021;373(6550):111–7.
Chen A, Liao S, Cheng M, Ma K, Wu L, Lai Y, et al. Spatiotemporal transcriptomic atlas of mouse organogenesis using DNA nanoball-patterned arrays. Cell. 2022;185(10):1777–92 e21.
Fu X, Sun L, Dong R, Chen JY, Silakit R, Condon LF, et al. Polony gels enable amplifiable DNA stam** and spatial transcriptomics of chronic pain. Cell. 2022;185(24):4621–33.e17.
Russell AJC, Weir JA, Nadaf NM, Shabet M, Kumar V, Kambhampati S, et al. Slide-tags enables single-nucleus barcoding for multimodal spatial genomics. Nature. 2024;625(7993):101–9.
Ma C, Yang C, Peng A, Sun T, Ji X, Mi J, et al. Pan-cancer spatially resolved single-cell analysis reveals the crosstalk between cancer-associated fibroblasts and tumor microenvironment. Mol Cancer. 2023;22(1):170.
Xun Z, Ding X, Zhang Y, Zhang B, Lai S, Zou D, et al. Reconstruction of the tumor spatial microenvironment along the malignant-boundary-nonmalignant axis. Nat Commun. 2023;14(1):933.
He S, ** Y, Nazaret A, Shi L, Chen X, Rampersaud S, et al. Starfysh integrates spatial transcriptomic and histologic data to reveal heterogeneous tumor–immune hubs. Nat Biotechnol. 2024:1–13.
Longo SK, Guo MG, Ji AL, Khavari PA. Integrating single-cell and spatial transcriptomics to elucidate intercellular tissue dynamics. Nat Rev Genet. 2021;22(10):627–44.
Andersson A, Larsson L, Stenbeck L, Salmen F, Ehinger A, Wu SZ, et al. Spatial deconvolution of HER2-positive breast cancer delineates tumor-associated cell type interactions. Nat Commun. 2021;12(1):6012.
Bassiouni R, Idowu MO, Gibbs LD, Robila V, Grizzard PJ, Webb MG, et al. Spatial transcriptomic analysis of a diverse patient cohort reveals a conserved architecture in triple-negative breast cancer. Cancer Res. 2023;83(1):34–48.
Monjo T, Koido M, Nagasawa S, Suzuki Y, Kamatani Y. Efficient prediction of a spatial transcriptomics profile better characterizes breast cancer tissue sections without costly experimentation. Sci Rep. 2022;12(1):4133.
Zhang Q, Abdo R, Iosef C, Kaneko T, Cecchini M, Han VK, et al. The spatial transcriptomic landscape of non-small cell lung cancer brain metastasis. Nat Commun. 2022;13(1):5983.
Zhu J, Fan Y, **ong Y, Wang W, Chen J, **a Y, et al. Delineating the dynamic evolution from preneoplasia to invasive lung adenocarcinoma by integrating single-cell RNA sequencing and spatial transcriptomics. Exp Mol Med. 2022;54(11):2060–76.
Wu R, Guo W, Qiu X, Wang S, Sui C, Lian Q, et al. Comprehensive analysis of spatial architecture in primary liver cancer. Sci Adv. 2021;7(51):eabg3750.
Hwang WL, Jagadeesh KA, Guo JA, Hoffman HI, Yadollahpour P, Reeves JW, et al. Single-nucleus and spatial transcriptome profiling of pancreatic cancer identifies multicellular dynamics associated with neoadjuvant treatment. Nat Genet. 2022;54(8):1178–91.
Liu Y, Xun Z, Ma K, Liang S, Li X, Zhou S, et al. Identification of a tumour immune barrier in the HCC microenvironment that determines the efficacy of immunotherapy. J Hepatol. 2023;78(4):770–82.
Ravi VM, Will P, Kueckelhaus J, Sun N, Joseph K, Salié H, et al. Spatially resolved multi-omics deciphers bidirectional tumor-host interdependence in glioblastoma. Cancer Cell. 2022;40(6):639–55.e13.
Ozato Y, Kojima Y, Kobayashi Y, Hisamatsu Y, Toshima T, Yonemura Y, et al. Spatial and single-cell transcriptomics decipher the cellular environment containing HLA-G+ cancer cells and SPP1+ macrophages in colorectal cancer. Cell Rep. 2023;42(1):111929.
Wu Y, Yang S, Ma J, Chen Z, Song G, Rao D, et al. Spatiotemporal immune landscape of colorectal cancer liver metastasis at single-cell level. Cancer Discov. 2022;12(1):134–53.
Wang Y, Chen D, Liu Y, Shi D, Duan C, Li J, et al. Multidirectional characterization of cellular composition and spatial architecture in human multiple primary lung cancers. Cell Death Dis. 2023;14(7):462.
Wang Y, Liu B, Min Q, Yang X, Yan S, Ma Y, et al. Spatial transcriptomics delineates molecular features and cellular plasticity in lung adenocarcinoma progression. Cell Discov. 2023;9(1):96.
Karras P, Bordeu I, Pozniak J, Nowosad A, Pazzi C, Van Raemdonck N, et al. A cellular hierarchy in melanoma uncouples growth and metastasis. Nature. 2022;610(7930):190–8.
Lin JR, Wang S, Coy S, Chen YA, Yapp C, Tyler M, et al. Multiplexed 3D atlas of state transitions and immune interaction in colorectal cancer. Cell. 2023;186(2):363–81 e19.
Gouin KH 3rd, Ing N, Plummer JT, Rosser CJ, Ben Cheikh B, Oh C, et al. An N-Cadherin 2 expressing epithelial cell subpopulation predicts response to surgery, chemotherapy and immunotherapy in bladder cancer. Nat Commun. 2021;12(1):4906.
Liu SQ, Gao ZJ, Wu J, Zheng HM, Li B, Sun S, et al. Single-cell and spatially resolved analysis uncovers cell heterogeneity of breast cancer. J Hematol Oncol. 2022;15(1):19.
Kumar V, Ramnarayanan K, Sundar R, Padmanabhan N, Srivastava S, Koiwa M, et al. Single-cell atlas of lineage states, tumor microenvironment, and subtype-specific expression programs in gastric cancer. Cancer Discov. 2022;12(3):670–91.
Sundar R, Liu DH, Hutchins GG, Slaney HL, Silva AN, Oosting J, et al. Spatial profiling of gastric cancer patient-matched primary and locoregional metastases reveals principles of tumour dissemination. Gut. 2021;70(10):1823–32.
Qi J, Sun H, Zhang Y, Wang Z, Xun Z, Li Z, et al. Single-cell and spatial analysis reveal interaction of FAP(+) fibroblasts and SPP1(+) macrophages in colorectal cancer. Nat Commun. 2022;13(1):1742.
Van de Velde LA, Allen EK, Crawford JC, Wilson TL, Guy CS, Russier M, et al. Neuroblastoma formation requires unconventional CD4 T cells and arginase-1-dependent myeloid cells. Cancer Res. 2021;81(19):5047–59.
Massalha H, Bahar Halpern K, Abu-Gazala S, Jana T, Massasa EE, Moor AE, et al. A single cell atlas of the human liver tumor microenvironment. Mol Syst Biol. 2020;16(12):e9682.
Sharma A, Seow JJW, Dutertre CA, Pai R, Bleriot C, Mishra A, et al. Onco-fetal reprogramming of Endothelial cells drives immunosuppressive macrophages in hepatocellular carcinoma. Cell. 2020;183(2):377–94 e21.
Stur E, Corvigno S, Xu M, Chen K, Tan Y, Lee S, et al. Spatially resolved transcriptomics of high-grade serous ovarian carcinoma. iScience. 2022;25(3):103923.
Moncada R, Barkley D, Wagner F, Chiodin M, Devlin JC, Baron M, et al. Integrating microarray-based spatial transcriptomics and single-cell RNA-seq reveals tissue architecture in pancreatic ductal adenocarcinomas. Nat Biotechnol. 2020;38(3):333–42.
Brady L, Kriner M, Coleman I, Morrissey C, Roudier M, True LD, et al. Inter- and intra-tumor heterogeneity of metastatic prostate cancer determined by digital spatial gene expression profiling. Nat Commun. 2021;12(1):1426.
Ji AL, Rubin AJ, Thrane K, Jiang S, Reynolds DL, Meyers RM, et al. Multimodal analysis of composition and spatial architecture in human squamous cell carcinoma. Cell. 2020;182(2):497–514 e22.
Grunwald BT, Devisme A, Andrieux G, Vyas F, Aliar K, McCloskey CW, et al. Spatially confined sub-tumor microenvironments in pancreatic cancer. Cell. 2021;184(22):5577–92 e18.
Barkley D, Moncada R, Pour M, Liberman DA, Dryg I, Werba G, et al. Cancer cell states recur across tumor types and form specific interactions with the tumor microenvironment. Nat Genet. 2022;54(8):1192–201.
Zhang T-L, **a C, Zheng B-W, Hu H-H, Jiang L-X, Escobar D, et al. Integrating single-cell and spatial transcriptomics reveals endoplasmic reticulum stress-related CAF subpopulations associated with chordoma progression. Neuro Oncol. 2023;26(2):295–308.
Gracia Villacampa E, Larsson L, Mirzazadeh R, Kvastad L, Andersson A, Mollbrink A, et al. Genome-wide spatial expression profiling in formalin-fixed tissues. Cell Genom. 2021;1(3):100065.
Erickson A, He M, Berglund E, Marklund M, Mirzazadeh R, Schultz N, et al. Spatially resolved clonal copy number alterations in benign and malignant tissue. Nature. 2022;608(7922):360–7.
Meylan M, Petitprez F, Becht E, Bougouin A, Pupier G, Calvez A, et al. Tertiary lymphoid structures generate and propagate anti-tumor antibody-producing plasma cells in renal cell cancer. Immunity. 2022;55(3):527–41 e5.
Wang F, Long J, Li L, Wu Z-X, Da T-T, Wang X-Q, et al. Single-cell and spatial transcriptome analysis reveals the cellular heterogeneity of liver metastatic colorectal cancer. Sci Adv. 2023;9(24):eadf5464.
Garbarino O, Lambroia L, Basso G, Marrella V, Franceschini B, Soldani C, et al. Spatial resolution of cellular senescence dynamics in human colorectal liver metastasis. Aging Cell. 2023;22(7):e13853.
Liu HT, Chen SY, Peng LL, Zhong L, Zhou L, Liao SQ, et al. Spatially resolved transcriptomics revealed local invasion-related genes in colorectal cancer. Front Oncol. 2023;13:1089090.
Fatemi M, Feng E, Sharma C, Azher Z, Goel T, Ramwala O, et al. Inferring spatial transcriptomics markers from whole slide images to characterize metastasis-related spatial heterogeneity of colorectal tumors: a pilot study. J Pathol Inform. 2023;14:100308.
Arora R, Cao C, Kumar M, Sinha S, Chanda A, McNeil R, et al. Spatial transcriptomics reveals distinct and conserved tumor core and edge architectures that predict survival and targeted therapy response. Nat Commun. 2023;14(1):5029.
Ferri-Borgogno S, Zhu Y, Sheng J, Burks JK, Gomez JA, Wong KK, et al. Spatial transcriptomics depict ligand-receptor cross-talk heterogeneity at the tumor-stroma interface in long-term ovarian cancer survivors. Can Res. 2023;83(9):1503–16.
Larroquette M, Guegan JP, Besse B, Cousin S, Brunet M, Le Moulec S, et al. Spatial transcriptomics of macrophage infiltration in non-small cell lung cancer reveals determinants of sensitivity and resistance to anti-PD1/PD-L1 antibodies. J Immunother Cancer. 2022;10(5):e003890.
Ferri-Borgogno S, Burks JK, Seeley EH, McKee TD, Stolley DL, Basi AV, et al. Molecular, metabolic, and subcellular map** of the tumor immune microenvironment via 3D targeted and non-targeted multiplex multi-omics analyses. Cancers (Basel). 2024;16(5):846.
Mbeunkui F, Johann DJ Jr. Cancer and the tumor microenvironment: a review of an essential relationship. Cancer Chemother Pharmacol. 2009;63(4):571–82.
Vitale I, Shema E, Loi S, Galluzzi L. Intratumoral heterogeneity in cancer progression and response to immunotherapy. Nat Med. 2021;27(2):212–24.
Marusyk A, Janiszewska M, Polyak K. Intratumor heterogeneity: the Rosetta stone of therapy resistance. Cancer Cell. 2020;37(4):471–84.
Tabassum DP, Polyak K. Tumorigenesis: it takes a village. Nat Rev Cancer. 2015;15(8):473–83.
Galeano Nino JL, Wu H, LaCourse KD, Kempchinsky AG, Baryiames A, Barber B, et al. Effect of the intratumoral microbiota on spatial and cellular heterogeneity in cancer. Nature. 2022;611(7937):810–7.
Ni Z, Prasad A, Chen S, Halberg RB, Arkin LM, Drolet BA, et al. SpotClean adjusts for spot swap** in spatial transcriptomics data. Nat Commun. 2022;13(1):2971.
Wang Y, Song B, Wang S, Chen M, **e Y, **ao G, et al. Sprod for de-noising spatially resolved transcriptomics data based on position and image information. Nat Methods. 2022;19(8):950–8.
Wang L, Maletic-Savatic M, Liu Z. Region-specific denoising identifies spatial co-expression patterns and intra-tissue heterogeneity in spatially resolved transcriptomics data. Nat Commun. 2022;13(1):6912.
Song T, Broadbent C, Kuang R. GNTD: reconstructing spatial transcriptomes with graph-guided neural tensor decomposition informed by spatial and functional relations. Nat Commun. 2023;14(1):8276.
Zhao Y, Wang K, Hu G. DIST: spatial transcriptomics enhancement using deep learning. Brief Bioinform. 2023;24(2):bbad013.
Zhao E, Stone MR, Ren X, Guenthoer J, Smythe KS, Pulliam T, et al. Spatial transcriptomics at subspot resolution with BayesSpace. Nat Biotechnol. 2021;39(11):1375–84.
Bergenstrahle L, He B, Bergenstrahle J, Abalo X, Mirzazadeh R, Thrane K, et al. Super-resolved spatial transcriptomics by deep data fusion. Nat Biotechnol. 2022;40(4):476–9.
Hua Y, Zhang Y, Guo Z, Bian S, Zhang Y. ImSpiRE: Image feature-aided spatial resolution enhancement method. bioRxiv. 2023:2023.05.04.539342.
Hu J, Coleman K, Zhang D, Lee EB, Kadara H, Wang L, et al. Deciphering tumor ecosystems at super resolution from spatial transcriptomics with TESLA. Cell Syst. 2023;14(5):404–17.
Hafemeister C, Satija R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 2019;20(1):296.
Svensson V, Teichmann SA, Stegle O. SpatialDE: identification of spatially variable genes. Nat Methods. 2018;15(5):343–6.
Sun S, Zhu J, Zhou X. Statistical analysis of spatial expression patterns for spatially resolved transcriptomic studies. Nat Methods. 2020;17(2):193–200.
Edsgard D, Johnsson P, Sandberg R. Identification of spatial expression trends in single-cell gene expression data. Nat Methods. 2018;15(5):339–42.
Zhang K, Feng W, Wang P. Identification of spatially variable genes with graph cuts. Nat Commun. 2022;13(1):5488.
Zhu J, Sun S, Zhou X. SPARK-X: non-parametric modeling enables scalable and robust detection of spatial expression patterns for large spatial transcriptomic studies. Genome Biol. 2021;22(1):184.
Zhang C, Dong K, Aihara K, Chen L, Zhang S. STAMarker: determining spatial domain-specific variable genes with saliency maps in deep learning. Nucleic Acids Res. 2023;51(20):e103.
Pham D, Tan X, Balderson B, Xu J, Grice LF, Yoon S, et al. Robust map** of spatiotemporal trajectories and cell-cell interactions in healthy and diseased tissues. Nat Commun. 2023;14(1):7739.
Dries R, Zhu Q, Dong R, Eng CL, Li H, Liu K, et al. Giotto: a toolbox for integrative analysis and visualization of spatial expression data. Genome Biol. 2021;22(1):78.
Yang Y, Shi X, Liu W, Zhou Q, Chan Lau M, Chun Tatt Lim J, et al. SC-MEB: spatial clustering with hidden Markov random field using empirical Bayes. Brief Bioinform. 2022;23(1):bbab466.
Liu W, Liao X, Yang Y, Lin H, Yeong J, Zhou X, et al. Joint dimension reduction and clustering analysis of single-cell RNA-seq and spatial transcriptomics data. Nucleic Acids Res. 2022;50(12):e72.
Li Z, Zhou X. BASS: multi-scale and multi-sample analysis enables accurate cell type clustering and spatial domain detection in spatial transcriptomic studies. Genome Biol. 2022;23(1):168.
Hu J, Li X, Coleman K, Schroeder A, Ma N, Irwin DJ, et al. SpaGCN: Integrating gene expression, spatial location and histology to identify spatial domains and spatially variable genes by graph convolutional network. Nat Methods. 2021;18(11):1342–51.
Dong K, Zhang S. Deciphering spatial domains from spatially resolved transcriptomics with an adaptive graph attention auto-encoder. Nat Commun. 2022;13(1):1739.
Li J, Chen S, Pan X, Yuan Y, Shen H-B. Cell clustering for spatial transcriptomics data with graph neural networks. Nat Comput Sci. 2022;2(6):399–408.
Long Y, Ang KS, Li M, Chong KLK, Sethi R, Zhong C, et al. Spatially informed clustering, integration, and deconvolution of spatial transcriptomics with GraphST. Nat Commun. 2023;14(1):1155.
Elosua-Bayes M, Nieto P, Mereu E, Gut I, Heyn H. SPOTlight: seeded NMF regression to deconvolute spatial transcriptomics spots with single-cell transcriptomes. Nucleic Acids Res. 2021;49(9):e50.
Dong R, Yuan GC. SpatialDWLS: accurate deconvolution of spatial transcriptomic data. Genome Biol. 2021;22(1):145.
Kleshchevnikov V, Shmatko A, Dann E, Aivazidis A, King HW, Li T, et al. Cell 2location maps fine-grained cell types in spatial transcriptomics. Nat Biotechnol. 2022;40(5):661–71.
Ma Y, Zhou X. Spatially informed cell-type deconvolution for spatial transcriptomics. Nat Biotechnol. 2022;40(9):1349–59.
Lopez R, Li B, Keren-Shaul H, Boyeau P, Kedmi M, Pilzer D, et al. DestVI identifies continuums of cell types in spatial transcriptomics data. Nat Biotechnol. 2022;40(9):1360–9.
Sun D, Liu Z, Li T, Wu Q, Wang C. STRIDE: accurately decomposing and integrating spatial transcriptomics using single-cell RNA sequencing. Nucleic Acids Res. 2022;50(7):e42.
Cable DM, Murray E, Zou LS, Goeva A, Macosko EZ, Chen F, et al. Robust decomposition of cell type mixtures in spatial transcriptomics. Nat Biotechnol. 2022;40(4):517–26.
Miller BF, Huang F, Atta L, Sahoo A, Fan J. Reference-free cell type deconvolution of multi-cellular pixel-resolution spatially resolved transcriptomics data. Nat Commun. 2022;13(1):2339.
Yang CX, Sin DD, Ng RT. SMART: reference-free deconvolution for spatial transcriptomics using marker-gene-assisted topic models. bioRxiv. 2023:2023.06. 20.545793.
Ru B, Huang J, Zhang Y, Aldape K, Jiang P. Estimation of cell lineages in tumors from spatial transcriptomics data. Nat Commun. 2023;14(1):568.
Wei R, He S, Bai S, Sei E, Hu M, Thompson A, et al. Spatial charting of single-cell transcriptomes in tissues. Nat Biotechnol. 2022;40(8):1190–9.
Vahid MR, Brown EL, Steen CB, Zhang W, Jeon HS, Kang M, et al. High-resolution alignment of single-cell and spatial transcriptomes with CytoSPACE. Nat Biotechnol. 2023;41(11):1543–8.
Wan X, **ao J, Tam SST, Cai M, Sugimura R, Wang Y, et al. Integrating spatial and single-cell transcriptomics data using deep generative models with SpatialScope. Nat Commun. 2023;14(1):7848.
Song Q, Su J. DSTG: deconvoluting spatial transcriptomics data through graph-based artificial intelligence. Brief Bioinform. 2021;22(5):bbaa414.
Efremova M, Vento-Tormo M, Teichmann SA, Vento-Tormo R. Cell PhoneDB: inferring cell–cell communication from combined expression of multi-subunit ligand–receptor complexes. Nat Protoc. 2020;15(4):1484–506.
** S, Guerrero-Juarez CF, Zhang L, Chang I, Ramos R, Kuan CH, et al. Inference and analysis of cell-cell communication using Cell Chat. Nat Commun. 2021;12(1):1088.
** S, Plikus MV, Nie Q. CellChat for systematic analysis of cell-cell communication from single-cell and spatially resolved transcriptomics. bioRxiv. 2023:2023.11.05.565674.
Cang Z, Nie Q. Inferring spatial and signaling relationships between cells from single cell transcriptomic data. Nat Commun. 2020;11(1):2084.
Cang Z, Zhao Y, Almet AA, Stabell A, Ramos R, Plikus MV, et al. Screening cell-cell communication in spatial transcriptomics via collective optimal transport. Nat Methods. 2023;20(2):218–28.
Xu H, Wang S, Fang M, Luo S, Chen C, Wan S, et al. SPACEL: deep learning-based characterization of spatial transcriptome architectures. Nat Commun. 2023;14(1):7603.
Zhou X, Dong K, Zhang S. Integrating spatial transcriptomics data across different conditions, technologies and developmental stages. Nat Comput Sci. 2023;3(10):894–906.
Zeira R, Land M, Strzalkowski A, Raphael BJ. Alignment and integration of spatial transcriptomics data. Nat Methods. 2022;19(5):567–75.
Liu X, Zeira R, Raphael BJ. Partial alignment of multislice spatially resolved transcriptomics data. Genome Res. 2023;33(7):1124–32.
**a CR, Cao ZJ, Tu XM, Gao G. Spatial-linked alignment tool (SLAT) for aligning heterogenous slices. Nat Commun. 2023;14(1):7236.
Wang G, Zhao J, Yan Y, Wang Y, Wu AR, Yang C. Construction of a 3D whole organism spatial atlas by joint modelling of multiple slices with deep neural networks. Nat Machine Intell. 2023;5(11):1200–13.
Saiselet M, Rodrigues-Vitória J, Tourneur A, Craciun L, Spinette A, Larsimont D, et al. Transcriptional output, cell-type densities, and normalization in spatial transcriptomics. J Mol Cell Biol. 2020;12(11):906–8.
Li B, Zhang W, Guo C, Xu H, Li L, Fang M, et al. Benchmarking spatial and single-cell transcriptomics integration methods for transcript distribution prediction and cell type deconvolution. Nat Methods. 2022;19(6):662–70.
Li H, Zhou J, Li Z, Chen S, Liao X, Zhang B, et al. A comprehensive benchmarking with practical guidelines for cellular deconvolution of spatial transcriptomics. Nat Commun. 2023;14(1):1548.
Garcia-Alonso L, Handfield LF, Roberts K, Nikolakopoulou K, Fernando RC, Gardner L, et al. Map** the temporal and spatial dynamics of the human endometrium in vivo and in vitro. Nat Genet. 2021;53(12):1698–711.
Heming M, Haessner S, Wolbert J, Lu IN, Li X, Brokinkel B, et al. Intratumor heterogeneity and T cell exhaustion in primary CNS lymphoma. Genome Med. 2022;14(1):109.
Xu GJ, Loberg MA, Gallant JN, Sheng Q, Chen SC, Lehmann BD, et al. Molecular signature incorporating the immune microenvironment enhances thyroid cancer outcome prediction. Cell Genom. 2023;3(10):100409.
Wang N, Li X, Wang R, Ding Z. Spatial transcriptomics and proteomics technologies for deconvoluting the tumor microenvironment. Biotechnol J. 2021;16(9):e2100041.
Chaurand P, Stoeckli M, Caprioli RM. Direct profiling of proteins in biological tissue sections by MALDI mass spectrometry. Anal Chem. 1999;71(23):5263–70.
Angelo M, Bendall SC, Finck R, Hale MB, Hitzman C, Borowsky AD, et al. Multiplexed ion beam imaging of human breast tumors. Nat Med. 2014;20(4):436–42.
Giesen C, Wang HA, Schapiro D, Zivanovic N, Jacobs A, Hattendorf B, et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat Methods. 2014;11(4):417–22.
Goltsev Y, Samusik N, Kennedy-Darling J, Bhate S, Hale M, Vazquez G, et al. Deep profiling of mouse splenic architecture with CODEX multiplexed imaging. Cell. 2018;174(4):968–81.
Lin JR, Fallahi-Sichani M, Sorger PK. Highly multiplexed imaging of single cells using a high-throughput cyclic immunofluorescence method. Nat Commun. 2015;6:8390.
Petras D, Jarmusch AK, Dorrestein PC. From single cells to our planet-recent advances in using mass spectrometry for spatially resolved metabolomics. Curr Opin Chem Biol. 2017;36:24–31.
Deng Y, Bartosovic M, Ma S, Zhang D, Kukanja P, **ao Y, et al. Spatial profiling of chromatin accessibility in mouse and human tissues. Nature. 2022;609(7926):375–83.
Llorens-Bobadilla E, Zamboni M, Marklund M, Bhalla N, Chen X, Hartman J, et al. Solid-phase capture and profiling of open chromatin by spatial ATAC. Nat Biotechnol. 2023;41(8):1085–8.
Engblom C, Thrane K, Lin Q, Andersson A, Toosi H, Chen X, et al. Spatial transcriptomics of B cell and T cell receptors reveals lymphocyte clonal dynamics. Science. 2023;382(6675):eadf8486.
Zhao T, Chiang ZD, Morriss JW, LaFave LM, Murray EM, Del Priore I, et al. Spatial genomics enables multi-modal study of clonal heterogeneity in tissues. Nature. 2022;601(7891):85–91.
Liu Y, DiStasio M, Su G, Asashima H, Enninful A, Qin X, et al. High-plex protein and whole transcriptome co-map** at cellular resolution with spatial CITE-seq. Nature Biotechnol. 2023;41(10):1405–9.
Zeng H, Huang J, Zhou H, Meilandt WJ, Dejanovic B, Zhou Y, et al. Integrative in situ map** of single-cell transcriptional states and tissue histopathology in a mouse model of Alzheimer’s disease. Nat Neurosci. 2023;26(3):430–46.
Liu S, Iorgulescu JB, Li S, Borji M, Barrera-Lopez IA, Shanmugam V, et al. Spatial maps of T cell receptors and transcriptomes reveal distinct immune niches and interactions in the adaptive immune response. Immunity. 2022;55(10):1940–52 e5.
Eid J, Fehr A, Gray J, Luong K, Lyle J, Otto G, et al. Real-time DNA sequencing from single polymerase molecules. Science. 2009;323(5910):133–8.
Branton D, Deamer DW, Marziali A, Bayley H, Benner SA, Butler T, et al. The potential and challenges of nanopore sequencing. Nat Biotechnol. 2008;26(10):1146–53.
Liao Y, Liu Z, Zhang Y, Lu P, Wen L, Tang F. High-throughput and high-sensitivity full-length single-cell RNA-seq analysis on third-generation sequencing platform. Cell Discov. 2023;9(1):5.
Singh M, Al-Eryani G, Carswell S, Ferguson JM, Blackburn J, Barton K, et al. High-throughput targeted long-read single cell sequencing reveals the clonal and transcriptional landscape of lymphocytes. Nat Commun. 2019;10(1):3120.
**a Y, ** Z, Zhang C, Ouyang L, Dong Y, Li J, et al. TAGET: a toolkit for analyzing full-length transcripts from long-read sequencing. Nat Commun. 2023;14(1):5935.
Kovaka S, Zimin AV, Pertea GM, Razaghi R, Salzberg SL, Pertea M. Transcriptome assembly from long-read RNA-seq alignments with StringTie2. Genome Biol. 2019;20(1):278.
Yan L, Sun X. Benchmarking and integration of methods for deconvoluting spatial transcriptomic data. Bioinformatics. 2023;39(1):btac805.
Sang-aram C, Browaeys R, Seurinck R, Saeys Y. Spotless: a reproducible pipeline for benchmarking cell type deconvolution in spatial transcriptomics. bioRxiv. 2023:2023.03. 22.533802.
Yuan Z, Zhao F, Lin S, Zhao Y, Yao J, Cui Y, et al. Benchmarking spatial clustering methods with spatially resolved transcriptomics data. Nat Methods. 2024;21(4):712–22.
Charitakis N, Salim A, Piers AT, Watt KI, Porrello ER, Elliott DA, et al. Disparities in spatially variable gene calling highlight the need for benchmarking spatial transcriptomics methods. Genome Biol. 2023;24(1):209.
** Z, Huang W, Shen N, Li J, Wang X, Dong J, et al. Single-cell gene fusion detection by scFusion. Nat Commun. 2022;13(1):1084.
Quinones-Valdez G, Fu T, Chan TW, **ao X. scAllele: a versatile tool for the detection and analysis of variants in scRNA-seq. Sci Adv. 2022;8(35):eabn6398.
Yuan Z, Pan W, Zhao X, Zhao F, Xu Z, Li X, et al. SODB facilitates comprehensive exploration of spatial omics data. Nat Methods. 2023;20(3):387–99.
Xu Z, Wang W, Yang T, Li L, Ma X, Chen J, et al. STOmicsDB: a comprehensive database for spatial transcriptomics data sharing, analysis and visualization. Nucleic Acids Res. 2024;52(D1):D1053–D61.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Key R&D Program of China [2020YFE0204200 to R.X.], the National Natural Science Foundation of China [12371286, 11971039 to R.X., 12201219 to J.M.], Sino-Russian Mathematics Center, Foundation of Qinglonghu laboratory, Shanghai Sailing Program (No. 21YF1410600 to J.M.), and Shanghai Key Program of Computational Biology (No. 23JS1400500, 23JS1400800 to J.M.).
Author information
Authors and Affiliations
Contributions
Conceptualization: **gsi Ming, Ruibin **; Writing-Original draft preparation: Siyuan Huang, Linkun Ouyang; Supervision: **gsi Ming, Ruibin **; Writing-Reviewing and Editing: Junjie Tang, Kun Qian, Xuanwei Chen, Zijie Xu, **gsi Ming, Ruibin **.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, S., Ouyang, L., Tang, J. et al. Spatial transcriptomics: a new frontier in cancer research. CCB 3, 13 (2024). https://doi.org/10.1007/s44272-024-00018-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44272-024-00018-8