Abstract
Background
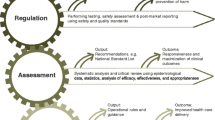
Cognizant of indispensable role as important health intervention tools, the global medical devices industry continues to bring new medical devices with varying degrees of technologies and complexities. Ensuring the safety, good performance and timely access of them have become challenging for regulatory authorities, particularly for develo** countries including Ethiopia. In Ethiopia, the role of the regulatory authority is complicated further because of the lack of specific policies. Medical devices regulation is still being dealt under drug policy.
Objectives
This study was aimed to assess the regulatory approval processes of medical devices in Ethiopia.
Methods
A mixed sequential explanatory study design was employed. Quantitative data were collected using a structured self-administered questionnaire and standard checklist; qualitative data were collected through in-depth interviews using a semi-structured guide.
Results
Retrospective trend analysis (2015 to 2018) indicated that 3,804 medical devices were registered in Ethiopia. Findings from the quantitative study indicated that 73.3% of regulatory experts had commendable knowledge on the medical devices regulatory system. However, gaps were identified in inspection and auditing (63.8%), practically understanding the system and procedures (24.3%), and having competencies in executing the critical core functions (6.9%). The top five challenges reported include (i) lack of capacity to assess dossiers (80.8%); (ii) lack of effective legislation (64.1%); (iii) provision of ambiguous feedback on deficiencies after dossier evaluations and delay in their communication (63.9%); (iv) long waiting time for approval (61.1%); and (v) lack of experienced and qualified staff (55.7%). In addition, the absence of a specific policy for medical device regulation presents a great hurdle.
Conclusion
Basic functional systems and procedures for the regulation of medical devices in Ethiopia are present. However, there are still gaps that are impeding effective regulation of medical devices especially for those with advanced features and complex-monitoring modalities.



Similar content being viewed by others
Data Availability
All basic data that are generated in the study are included in the manuscript. However, if additional more data are needed, the corresponding author Ayenew Ashenef can offer upon reasonable request.
References
WHO-ROEM. The role of medical devices and equipment in contemporary health care systems and services, Technical Discussions, 53rd Regional, World Health Organization, Regional Office for the Eastern and Mediterranean (WHO-ROEM), June 2006. https://applications.emro.who.int/docs/EM_RC53_Tech.Disc.2_en.pdf. Accessed 12 June 2021.
Sarah H, Maloney C, Phillips SD, et al. The evolving landscape of medical device regulation in east, central, and southern Africa. Glob Health Sci Pract. 2021;9:136–48.
GSMA-mHealth. Understanding medical device regulation for mHealth: a guide for mobile operators, GSMA connected living programme, Feb 2012. https://www.gsma.com/iot/wp-content/uploads/2012/03/gsmaunderstandingmedicaldeviceregulationformhealthreport1.pdf. Accessed 8 Feb 2021.
Peter L, Hajek L, Maresova P, et al. Medical devices: Regulation, risk classification, and open innovation. J Open Innov Technol Mark Complex. 2020;6:42. https://doi.org/10.3390/joitmc6020042.
WHO. Medical devices: Managing the mismatch: an outcome of the priority medical devices project. Geneva: World Health Organization; 2017. http://apps.who.int/iris/bitstream/10665/44407/1/9789241564045_eng.pdf. Accessed 07 June 2019.
WHO. Medical Device Regulations: Global Overview and Guiding Principles. Geneva: World Health Organization (WHO), Geneva; 2018. http://apps.who.int/iris/bitstream/10665/44407/1/978924156773. Accessed 23 Mar 2023.
Rados C. Medical device and radiological health regulations come of age. FDA Consumer Magazine, Centennial Edition/Jan–Feb 2006. https://www.fda.gov/files/about%20fda/published/Medical-Device-and-Radiological-Health-Regulations-Come-of-Age.pdf. Accessed 24 Mar 2021.
Fries R. Reliable Design of Medical Devices, 2nd ed. Boca Raton, FL: CRC. http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/Posmarket
WHO. Global model regulatory framework for medical devices including in vitro diagnostic medical devices, WHO Medical Device Technical Series, 2017. https://apps.who.int/iris/bitstream/handle/10665/255177/9789241512350-eng.pdf. Accessed 16 May 2021.
FDRE. Drug Administration and Control Proclamation (Proc. No. 176/1999), Federal Negarit Gazeta, 5th Year No. 60, Federal Democratic Republic of Ethiopia (EDRE), ADDIS ABABA, 29th June 1999: http://www.efda.gov.et/publication/drug-administration-and-control-proclamation-no-176-99/. Accessed 23 Mar 2023.
FDRE (2019). Food and Medicine Administration Proclamation (No.1112/2019), Federal Negarit Gazeta, 25th Year No. 39, Federal Democratic Republic of Ethiopia (EDRE), ADDIS ABABA, 28 February 2019: https://ethionab.org/wp-content/uploads/2022/09/1112_2019_A_PROCLAMATION_TO_PROVIDE_FOR_FOOD_AND_MEDICINE_ADMINISTRATION_.pdf. Accessed 23 Mar 2023.
Kaggwa LS, Lemeshow S. Sample size determination in health studies: a practical manual. World Health Organization, 1991. http://apps.who.int/iris/bitstream/handle/10665/40062/9241544058_(p1-p22).pdf?sequence=1. Accessed 24 Mar 2021.
EFDA. Guideline for classification of medical devices other than IVD medical devices, Ethiopian Food and Drug Authority (EFDA), Addis Ababa, July 2021. http://www.fmhaca.gov.et/wp-content/uploads/2021/07/Guideline-for-Classification-of-Medical-devices-other-than-IVD-Medical-devices_EFDA.pdf. Accessed 25 May 2022.
Rugera SP, McNerney R, Poon AK, et al. Regulation of medical diagnostics and medical devices in the East African community partner states. BMC Health Serv Res. 2014;14:1–7.
Contardi M. Changes in the medical device’s regulatory framework and its impact on the medical device’s industry: from the medical device directives to the medical device regulations. Erasmus L Rev. 2019;12:166. https://doi.org/10.5553/ELR.000139.
Guerra-Bretaña RM, Flórez-Rendón AL. Impact of regulations on innovation in the field of medical devices. Res Biomed Eng. 2018;34:356–67.
Bergsland J, Elle OJ, Fosse E. Barriers to medical device innovation. Med Devices Evid Res. 2014;7:205. https://doi.org/10.2147/MDER.S43369.
Gullane, E. An analysis of medical device registration and market access in china. Doctoral Research Dissertation, Institute of Technology, Sligo, 2017. https://research.thea.ie/bitstream/handle/20.500.12065/2235/Elaine%20Gullane_DissertationFinal%20-%20pdf_Redacted.pdf?sequence=4&isAllowed=y. Accessed 12 June 2022.
Ademe BW, Tebeje B, Molla A. Availability and utilization of medical devices in Jimma Zone Hospitals. Southwest Ethiopia BMC Health Serv Res. 2016;16:1–10.
GHTF. Registration of manufacturers and other parties and listing of medical devices, final document. Global Harmonization Task Force (GHTF). August 2010. https://www.imdrf.org/sites/default/files/docs/ghtf/final/sg1/technical-docs/ghtf-sg1-n065-listing-of-medical-devices-100827.pdf. Accessed 12 June 2022.
GHTF. Principles of conformity assessment for medical devices (GHTF/SG1/N40:2006). Global Harmonization Task Force (GHTF), November 2012. https://www.imdrf.org/sites/default/files/docs/ghtf/final/sg1/technical-docs/ghtf-sg1-n78-2012-conformity-assessment-medical-devices-121102.pdf. Accessed 12 June 2022.
EU. Regulation of the European Parliament and the Council on Medical Devices Amending Directive 2017/745. April 2017. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0745. Accessed 12 June 2022.
Saini KS, Kaushik A, Anil B, et al. Harmonized medical device regulation: need, challenges, and risks of not harmonizing the regulation in Asia. J Young Pharm. 2010;2:101–6.
Ramakrishna S, Tian L, Wang C, et al. Medical devices: regulations, standards and practices. Elsevier Science; 2015. p. 525.
Maresova P, Penhaker M, Selamat A, et al. The potential of medical device industry in technological and economical context. Ther Clin Risk Manag. 2015;11:1505–14.
O’brien C. Investigation into the challenges encountered by regulatory affairs professionals working in the Medtech industry, Masters Thesis, Atlantic Techological University, Sligo, 21 Aug 2017. https://research.thea.ie/handle/20.500.12065/2236. Accessed 12 June 2022.
Kaule S, Bock A, Dierke A, et al. Medical device regulation and current challenges for the implementation of new technologies. Curr Dir Biomed Eng. 2020;6:334–7.
Matovu B, Takuwa M, Mpaata CN, et al. Review of investigational medical devices’ clinical trials and regulations in Africa as a benchmark for new innovations. Front Med Technol. 2022;4:952767. https://doi.org/10.3389/fmedt.2022.952767.
Funding
Addis Ababa University (AAU) supported this research through graduate students support programme while the Ethiopian Food and Drug Authority offered study sponsorship to Kebede Fufa.
Author information
Authors and Affiliations
Contributions
Conceptualization, study tools and design: KF, TM, AA. Data collection and acquisition: KF. Data Interpretation and analysis: KF, TM, AA. Drafting of the Manuscript: KF, TM. Manuscript reviewing and editing: AA, TM. Supervision: AA, TM. All authors had read and approved the final version of the manuscript submitted.
Corresponding author
Ethics declarations
Conflict of interest
There is not any conflict of interest to declare by the Authors. Although AAU and EFDA supported the study, both organizations did not have any role or influence in study design, data interpretation and writing of this manuscript. Informed consent was obtained from the study participants. The study was ethically cleared before commencement.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fufa, K., Marew, T. & Ashenef, A. Assessment of the Regulatory Approval Process of Medical Devices in Ethiopia: A Mixed Sequential Explanatory Study. Ther Innov Regul Sci 57, 987–996 (2023). https://doi.org/10.1007/s43441-023-00534-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43441-023-00534-0