Abstract
Background
The Canadian guideline on bioequivalence allows identification of outliers through for example studentized residuals, and it explicitly accepts exclusion of subject data when their studentized residuals reach a certain magnitude. The guideline also requires that the type I errors (patient’s risk, chance of declaring bioequivalence for a bioinequivalent product) be maintained at the 5% level. This manuscript investigates if the outlier removal procedure increases type I errors when alpha of 5% is used for construction of the confidence interval.
Methods
Numerical simulation is used.
Results
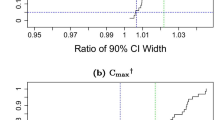
Patient’s risk may be inflated beyond 5% when the traditional 90% confidence interval is constructed. Maximum type I errors observed here are slightly above 7%. To circumvent the issue sponsors can either to abstain from outlier removal or find ways to adjust alpha levels downwards if the patient’s risk is to be preserved at the 5% level.
Conclusion
This manuscript is the first to create awareness of a potential inflation of the type I error when 90% confidence intervals are constructed as per Canadian guidance. The issue may not just affect patients in Canada but could also affect patients in the USA since the current rules allow American patients to import a 3 month supply of drugs for personal use and because the central administration is actively pursuing plans to “(…) facilitate the importation of certain prescription drugs that are approved in Canada”.


Similar content being viewed by others
References
United States Food and Drug Administration, Center for Drug Evaluation and Research. Statistical approaches to establishing bioequivalence. Guidance for industry: statistical approaches to establishing bioequivalence. 2001. https://www.fda.gov/media/70958/download
European Medicines Agency, Committee for Human Medicinal Products. Investigation of bioequivalence. CHMP CPMP/EWP/QWP/1401/98 Rev. 1. 2010. http://www.ema.europa.eu/ema/pages/includes/document/open_document.jsp?webContentId=WC500070039.
World Health Organization. Annex 7, Multisource (generic) pharmaceutical products: guidelines on registration requirements to establish interchangeability. WHO Technical Report Series No. 992, 2015. https://www.who.int/medicines/areas/quality_safety/quality_assurance/Annex7-TRS992.pdf.
Health Canada. Conduct and analysis of comparative bioavailability studies. February 2012, June 2018. https://www.canada.ca/content/dam/hc-sc/documents/services/drugs-health-products/drug-products/applications-submissions/guidance-documents/bioavailability-bioequivalence/conduct-analysis-comparative.pdf
United States Food and Drug Administration, Center for Drug Evaluation and Research. Approval package for ANDA 77-883, December 2007.
Zizou. Rules acc. to Health Canada, February, 2019. http://forum.bebac.at/mix_entry.php?id=19867#p19886
Huang Y, Ke BS. Influence analysis on crossover design experiment in bioequivalence studies. Pharm Stat. 2014;13(2):110–8.
Schall R, Endrenyi L, Ring A. Residuals and outliers in replicate design crossover studies. J Biopharm Stat. 2010;20(4):835–49.
Liao JJ. A new approach for outliers in a bioavailability/bioequivalence study. J Biopharm Stat. 2007;17(3):393–405.
Ramsay T, Elkum N. A comparison of four different methods for outlier detection in bioequivalence studies. J Biopharm Stat. 2005;15(1):43–52.
Wang W, Chow SC. Examining outlying subjects and outlying records in bioequivalence trials. J Biopharm Stat. 2003;13(1):43–56.
Ki FY, Liu JP, Wang W, Chow SC. The impact of outlying subjects on decision of bioequivalence. J Biopharm Stat. 1995;5(1):71–94.
Chow S-C, Liu J-P. Design and analysis of bioavailability and bioequivalence studies. 3rd ed. Boca Raton: Chapman & Hall; 2009.
Schuirmann DJ. A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm. 1987;15:657–80.
Potvin D, DiLiberti CE, Hauck WW, Parr AF, Schuirmann DJ, Smith RA. Sequential design approaches for bioequivalence studies with crossover designs. Pharm Stat. 2008;7:245–62.
United States Food and Drug Administration. Is it legal for me to personally import drugs? March 2018. https://www.fda.gov/about-fda/fda-basics/it-legal-me-personally-import-drugs
United States Food and Drug Administration. FDA takes actions to help lower U.S. prescription drug prices final rule, guidance fulfill plan for safe importation of certain drugs originally intended for foreign markets. September 2020. https://www.fda.gov/news-events/press-announcements/fda-takes-actions-help-lower-us-prescription-drug-prices
United States Food and Drug Administration. Importation of prescription drugs final rule. September 2020. https://www.hhs.gov/sites/default/files/importation-final-rule.pdf. Accessed 6 June 2021.
Acknowledgements
The author is grateful to the user “zizou” who aired their question on forum.bebac.at.
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Contributions
AF planned, executed, and reported this study as single author.
Corresponding author
Ethics declarations
Conflict of interest
The author is a consultant. Present and former clients include pharmacopeias, agencies, the World Health Organization, and private companies. This paper expresses no opinion or inference about agencies, organizations, companies, political parties, or medicinal products. The author declares no conflict of interest.
Rights and permissions
About this article
Cite this article
Fuglsang, A. Increased Patient’s Risk Associated with the Canadian Bioequivalence Guidance Due to Outlier Removal. Ther Innov Regul Sci 56, 168–172 (2022). https://doi.org/10.1007/s43441-021-00344-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43441-021-00344-2