Abstract
Background
There are few effective treatments for Candida biofilm-associated infections. The present study demonstrated changes in the expression of biofilm-associated genes in Candida albicans treated with magnetic iron oxide nanoparticles (denoted as nano-Fe3O4).
Methods
Nano-Fe3O4 was biologically synthesized using Bacillus licheniformis, Bacillus cereus, and Fusarium oxysporum. Additionally, the biologically synthesized nano-Fe3O4 was characterized by visual observation; ultraviolet–visible spectroscopy, scanning electron microscopy, X-ray diffraction spectroscopy, and Fourier transform infrared spectroscopy. The biologically synthesized nano-Fe3O4 was tested for growth and biofilm formation in C. albicans. Furthermore, quantitative real-time reverse transcriptase–polymerase chain reaction (RT-PCR) was used to study the inhibition of biofilm-associated genes in C. albicans treated with nano-Fe3O4.
Results
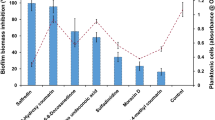
The production of biologically synthesized nano-Fe3O4 was confirmed using extensive characterization methods. The nano-Fe3O4 inhibited growth and biofilm formation. Nano-Fe3O4 exhibited growth inhibition with minimum inhibition concentrations (MICs) of 50 to 200 μg mL−1. The anti-biofilm effects of nano-Fe3O4 were shown by 2,3-bis (2-methoxy-4-nitro-5 sulfophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide (XTT) reduction assay, crystal violet staining, and light field microscopy. The gene expression results showed that the downregulation of BCR1, ALS1, ALS3, HWP1, and ECE1 genes inhibited the biofilm formation in C. albicans. ALS1 reduction was greater than others, with downregulation of 1375.83-, 1178.71-, and 768.47-fold at 2 × MIC, 1 × MIC, and ½ × MIC of nano-Fe3O4, respectively.
Conclusion
Biofilm-associated genes as potential molecular targets of nano-Fe3O4 in C. albicans may be an effective novel treatment strategy for biofilm-associated infections.



Similar content being viewed by others
Data availability
Data generated/analyzed during this study are included in the article and its supplementary information files or are available on request from the corresponding author.
Abbreviations
- ALS :
-
Agglutinin-like protein
- ANOVA:
-
Analysis of variance
- BCR1 :
-
Biofilm and cell wall regulator 1
- BRG1 :
-
Biofilm regulator 1
- cDNA:
-
Complementary DNA
- CDR1 :
-
Candida Drug resistance 1
- CEK1-MAPK:
-
Candida albicans extracellular signal-regulated kinase 1-Mitogen-activated protein kinase
- CV:
-
Crystal violet
- EAP1 :
-
Enhanced adherence to polystyrene 1
- ECE1 :
-
Extent of cell elongation 1
- EFG1 :
-
Enhanced filamentous growth protein 1
- ERG11 :
-
Ergosterol biosynthesis gene 11
- FAV2 :
-
Factor activated 2
- FCC:
-
Face-centered cubic
- FDA:
-
Food and drug administration
- FTIR:
-
Fourier transform infrared
- GM:
-
Geometric mean
- GSC1 :
-
Glucan synthase catalytic subunit 1
- HWP1 :
-
Hyphal-specific wall protein 1
- HYR1 :
-
Hyphally regulated 1
- ICDD:
-
International centre of diffraction data
- IFF :
-
Individual protein file family F
- MDR1 :
-
Multidrug resistance 1
- MFC:
-
Minimum fungicidal concentration
- MIC:
-
Minimum inhibition concentration
- MNN1 :
-
Mannosyl transferase 1
- MSB2 :
-
Multicopy suppressor of bud emergence 2
- NDT80 :
-
Non-dityrosine 80
- ORF:
-
Open reading frame
- PBS:
-
Phosphate-buffered saline
- PGA :
-
Putative glycosylphosphatidylinositol-anchored proteins
- Ras-cAMP-EFG1 :
-
Rat sarcoma- cyclic adenosine monophosphate-Enhanced filamentous growth protein 1
- RCF:
-
Relative centrifugal force
- ROB1 :
-
Regulator of biofilm 1
- RPM:
-
Revolutions per minute
- RPMI:
-
Roswell park memorial institute medium
- RT-PCR:
-
Real-time reverse transcriptase–Polymerase chain reaction
- SEM:
-
Scanning electron microscopy
- SIM1 :
-
Secreted beta-glucosidase 1
- TEC1 :
-
Transposon enhancement control 1
- UV–Vis:
-
Ultraviolet–visible
- XRD:
-
X-ray diffraction
- XTT:
-
2,3-Bis (2-methoxy-4-nitro-5 sulfophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide
References
Laha SS, Abdelhamid E, Arachchige MP, Kumar A, Dixit A. Ferroic ordering and charge-spin-lattice order coupling in Gd-doped Fe3O4 nanoparticles relaxor multiferroic system. J Am Ceram Soc. 2017;100:1534–41.
Nguyen MD, Tran HV, Xu S, Lee TR. Fe3O4 nanoparticles: structures, synthesis, magnetic properties, surface functionalization, and emerging applications. Appl Sci. 2021;11:11301.
Abbas HS, Krishnan A. Magnetic nanosystems as a therapeutic tool to combat pathogenic fungi. Adv Pharm Bull. 2020;10:512–23.
Ali A, Shah T, Ullah R, Zhou P, Guo M, Ovais M, et al. Review on recent progress in magnetic nanoparticles: synthesis, characterization, and diverse applications. Front Chem. 2021;9: 629054.
Ashraf H, Batool T, Anjum T, Illyas A, Li G, Naseem S, et al. Antifungal potential of green synthesized magnetite nanoparticles black coffee-magnetite nanoparticles against wilt infection by ameliorating enzymatic activity and gene expression in Solanum lycopersicum L. Front Microbiol. 2022;13: 754292.
Shah IH, Manzoor MA, Sabir IA, Ashraf M, Gulzar S, Chang L, et al. A green and environmental sustainable approach to synthesis the Mn oxide nanomaterial from Punica granatum leaf extracts and its in vitro biological applications. Environ Monit Assess. 2022;194:921.
Salem DM, Ismail MM, Aly-Eldeen MA. Biogenic synthesis and antimicrobial potency of iron oxide (Fe3O4) nanoparticles using algae harvested from the Mediterranean Sea. Egypt Egypt J Aquat Res. 2019;45:197–204.
Iqbal J, Abbasi BA, Ahmad R, Shahbaz A, Zahra SA, Kanwal S, et al. Biogenic synthesis of green and cost effective iron nanoparticles and evaluation of their potential biomedical properties. J Mol Struct. 2020;1199: 126979.
Biswas A, Vanlalveni C, Lalfakzuala R, Nath S, Rokhum L. Mikania mikrantha leaf extract mediated biogenic synthesis of magnetic iron oxide nanoparticles: characterization and its antimicrobial activity study. Mater Today Proc. 2021;42:1366–73.
Paluch E, Szperlik J, Lamch Ł, Wilk KA, Obłąk E. Biofilm eradication and antifungal mechanism of action against Candida albicans of cationic dicephalic surfactants with a labile linker. Sci Rep. 2021;11:8896.
Centers for Disease Control and Prevention. CDC antibiotic resistance threats in the United States, 2019. https://www.cdc.gov/drugresistance/biggest-threats. html. Accessed 20 April 2021.
Pohl CH. Recent advances and opportunities in the study of Candida albicans polymicrobial biofilms. Front Cell Infect Microbiol. 2022;12: 836379.
Naglik JR, Gaffen SL, Hube B. Candidalysin: discovery and function in Candida albicans infections. Curr Opin Microbiol. 2019;52:100–9.
Pereira R, dos Santos Fontenelle RO, de Brito EH, de Morais SM. Biofilm of Candida albicans: formation, regulation and resistance. J Appl Microbiol. 2021;131:11–22.
Atriwal T, Azeem K, Husain FM, Hussain A, Khan MN, Alajmi MF, et al. Mechanistic understanding of Candida albicans biofilm formation and approaches for its inhibition. Front Microbiol. 2021;12: 638609.
Fox EP, Bui CK, Nett JE, Hartooni N, Mui MC, Andes DR, et al. An Expanded regulatory network temporally controls Candida albicans biofilm formation. Mol Microbiol. 2015;96:1226–39.
Niemirowicz K, Durnaś B, Tokajuk G, Piktel E, Michalak G, Gu X, et al. Formulation and candidacidal activity of magnetic nanoparticles coated with cathelicidin LL-37 and ceragenin CSA-13. Sci Rep. 2017;7:4610.
Vera-González N, Shukla A. Advances in biomaterials for the prevention and disruption of Candida Biofilms. Front Microbiol. 2020;11:538602.
Zare-Khafri M, Alizadeh F, Nouripour-Sisakht S, Khodavandi A, Gerami M. Inhibitory effect of magnetic iron-oxide nanoparticles on the pattern of expression of lanosterol 14α-demethylase (ERG11) in fluconazole-resistant colonising isolate of Candida albicans. IET Nanobiotechnol. 2020;14:375–81.
Halbandge SD, Jadhav AK, Jangid PM, Shelar AV, Patil RH, Karuppayil SM. Molecular targets of biofabricated silver nanoparticles in Candida albicans. J Antibiot. 2019;72:640–4.
Sun L, Liao K, Wang D. Effects of magnolol and honokiol on adhesion, yeast-hyphal transition, and formation of biofilm by Candida albicans. PLoS ONE. 2015;10: e0117695.
Fazly A, Jain C, Dehner AC, Issi L, Lilly EA, Ali A, et al. Chemical screening identifies filastatin, a small molecule inhibitor of Candida albicans adhesion, morphogenesis, and pathogenesis. Proc Natl Acad Sci USA. 2013;110:13594–9.
Kalishwaralal K, Deepak V, Ramkumarpandian S, Nellaiah H, Sangiliyandi G. Extracellular biosynthesis of silver nanoparticles by the culture supernatant of Bacillus licheniformis. Mater Lett. 2008;62:4411–3.
Sundaram PA, Augustine R, Kannan M. Extracellular biosynthesis of iron oxide nanoparticles by Bacillus subtilis strains isolated from rhizosphere soil. Biotechnol Bioproc E. 2012;17:835–40.
Bharde A, Rautaray D, Bansal V, Ahmad A, Sarkar I, Yusuf SM, et al. Extracellular biosynthesis of magnetite using fungi. Small. 2006;2:135–41.
Pfaller MA, Sheehan DJ, Rex JH. Determination of fungicidal activities against yeasts and molds: lessons learned from bactericidal testing and the need for standardization. Clin Microbiol Rev. 2004;17:268–80.
Ganan M, Lorentzen SB, Agger JW, Heyward CA, Bakke O, Knutsen SH, et al. Antifungal activity of well-defined chito-oligosaccharide preparations against medically relevant yeasts. PLoS ONE. 2019;14: e0210208.
Khodavandi A, Harmal NS, Alizadeh F, Scully OJ, Sidik SM, Othman F, et al. Comparison between allicin and fluconazole in Candida albicans biofilm inhibition and in suppression of HWP1 gene expression. Phytomedicine. 2011;19:56–63.
De Chaumont F, Dallongeville S, Chenouard N, Hervé N, Pop S, Provoost T, et al. Icy: an open bioimage informatics platform for extended reproducible research. Nat Methods. 2012;9:690–6.
Rocha FA, Alves AM, Rocha MF, Cordeiro RD, Brilhante RS, Pinto AC, et al. Tumor necrosis factor prevents Candida albicans biofilm formation. Sci Rep. 2017;7:1206.
Goyard S, Knechtle P, Chauvel M, Mallet A, Prévost MC, Proux C, et al. The Yak1 kinase is involved in the initiation and maintenance of hyphal growth in Candida albicans. Mol Biol Cell. 2008;19:2251–66.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–8.
Cichoń M. Reporting statistical methods and outcome of statistical analyses in research articles. Pharmacol Rep. 2020;72:481–5.
Iconaru SL, Guégan R, Popa CL, Motelica-Heino M, Ciobanu CS, Predoi D. Magnetite (Fe3O4) nanoparticles as adsorbents for As and Cu removal. App Clay Sci. 2016;134:128–35.
Win TT, Khan S, Bo B, Zada S, Fu P. Green synthesis and characterization of Fe3O4 nanoparticles using Chlorella-K01 extract for potential enhancement of plant growth stimulating and antifungal activity. Sci Rep. 2021;11:21996.
Pistoia ES, Cosio T, Campione E, Pica F, Volpe A, Marino D, et al. All-trans retinoic acid effect on Candida albicans growth and biofilm formation. J Fungi. 2022;8:1049.
Jacinto MJ, Silva VC, Valladão DM, Souto RS. Biosynthesis of magnetic iron oxide nanoparticles: a review. Biotechnol Lett. 2021;43:1–12.
Kaur K, Sidhu AK. Green synthesis: an eco-friendly route for the synthesis of iron oxide nanoparticles. Front Nanotechnol. 2021;3: 655062.
Khan S, Akhtar N, Rehman SU, Shujah S, Rha ES, Jamil M. Biosynthesized iron oxide nanoparticles (Fe3O4 NPs) mitigate arsenic toxicity in rice seedlings. Toxics. 2020;9:2.
Seddighi NS, Salari S, Izadi AR. Evaluation of antifungal effect of ironoxide nanoparticles against different Candida species. IET Nanobiotechnol. 2017;11:883–8.
Adhikari A, Chhetri K, Acharya D, Pant B, Adhikari A. Green synthesis of iron oxide nanoparticles using Psidium guajava L. leaves extract for degradation of organic dyes and anti-microbial applications. Catalysts. 2022;12:1188.
Alangari A, Alqahtani MS, Mateen A, Kalam MA, Alshememry A, Ali R, et al. Iron oxide nanoparticles: preparation, characterization, and assessment of antimicrobial and anticancer activity. Adsorp Sci Technol. 2022;2022:1–9.
Attia NF, Abd El-Monaem EM, El-Aqapa HG, Elashery SE, Eltaweil AS, El Kady M, et al. Iron oxide nanoparticles and their pharmaceutical applications. Appl Surf Sci Adv. 2022;11: 100284.
Zou F, Xu J, Yuan L, Zhang Q, Jiang L. Recent progress on smart hydrogels for biomedicine and bioelectronics. Biosurf Biotribol. 2022;8:212–24.
Baptista PV, McCusker MP, Carvalho A, Ferreira DA, Mohan NM, Martins M, et al. Nano-strategies to fight multidrug resistant bacteria—“A Battle of the Titans.” Front Microbiol. 2018;9:1441.
Khalid HF, Tehseen B, Sarwar Y, Hussain SZ, Khan WS, Raza ZA, et al. Biosurfactant coated silver and iron oxide nanoparticles with enhanced anti-biofilm and anti-adhesive properties. J Hazard Mater. 2019;364:441–8.
Alherz FA, Negm WA, Elekhnawy E, El-Masry TA, Haggag EM, Alqahtani MJ, et al. Silver nanoparticles prepared using Encephalartos laurentianus de wild leaf extract have inhibitory activity against Candida albicans clinical isolates. J Fungi. 2022;8:1005.
Wunnoo S, Paosen S, Lethongkam S, Sukkurd R, Waen-ngoen T, Nuidate T, et al. Biologically rapid synthesized silver nanoparticles from aqueous Eucalyptus camaldulensis leaf extract: effects on hyphal growth, hydrolytic enzymes, and biofilm formation in Candida albicans. Biotechnol Bioeng. 2021;118:1597–611.
Judan Cruz KG, Alfonso ED, Fernando SID, Watanabe K. Candida albicans biofilm inhibition by ethnobotanicals and ethnobotanically-synthesized gold nanoparticles. Front Microbiol. 2021;12: 665113.
Yu Q, Li J, Zhang Y, Wang Y, Liu L, Li M. Inhibition of gold nanoparticles (AuNPs) on pathogenic biofilm formation and invasion to host cells. Sci Rep. 2016;6:26667.
Acknowledgements
The authors wish to thank the Islamic Azad University of Shiraz for infrastructure facilities. The results presented in this study are part of a PhD thesis (IR.IAU.SHIRAZ.REC.1401.034).
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
AK, FA, and NB: made substantial contributions to the conception and design of the study. MBE, AK, and FA: conducted the search, analyzed the data, and wrote the manuscript. All authors read and approved the final content.
Corresponding author
Ethics declarations
Conflict of interest
The authors have nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baghiat Esfahani, M., Khodavandi, A., Alizadeh, F. et al. Biofilm-associated genes as potential molecular targets of nano-Fe3O4 in Candida albicans. Pharmacol. Rep 75, 682–694 (2023). https://doi.org/10.1007/s43440-023-00467-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-023-00467-3