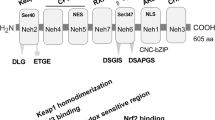
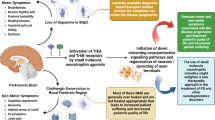
Abstract
Movement disorders are neurological conditions characterized by involuntary motor movements, such as dystonia, ataxia, chorea myoclonus, tremors, Huntington’s disease (HD), and Parkinson’s disease (PD). It is classified into two categories: hypokinetic and hyperkinetic movements. Globally, movement disorders are a major cause of death. The pathophysiological process is initiated by excessive ROS generation, mitochondrial dysfunction, neuroinflammation, and neurotransmitters imbalance that lead to motor dysfunction in PD and HD patients. Several endogenous targets including Nrf2 maintain oxidative balance in the body. Activation of Nrf2 signaling is regulated by the enzyme glycogen synthase kinase (GSK-3β). In the cytoplasm, inhibition of GSK-3β regulates cellular proliferation, homeostasis, and apoptotic process by stimulating the nuclear factor erythroid 2 (Nrf2) pathway which is involved in the elevation of the cellular antioxidant enzymes which controls the ROS generation. The activation of Nrf2 increases the expression of antioxidant response elements (ARE), such as (Hemeoxygenase-1) HO-1, which decreases excessive cellular stress, mitochondrial dysfunction, apoptosis, and neuronal degeneration, which is the major cause of motor dysfunction. The present review explores the GSK-3β-mediated neuroprotection in various movement disorders through the Nrf2/HO-1 antioxidant pathway. This review provides a link between GSK-3β and the Nrf2/HO-1 signaling pathway in the treatment of PD and HD. In addition to that it highlights various GSK-3β inhibitors and the Nrf2/HO-1 activators, which exert robust neuroprotection against motor disorders. Therefore, the present review will help in the discovery of new therapy for PD and HD patients.


Similar content being viewed by others
Data availability
Not applicable.
References
Murthy M, Cheng YY, Holton JL, Bettencourt C. Neurodegenerative movement disorders: an epigenetics perspective and promise for the future. Neuropathol Appl Neurobiol. 2021;47(7):897–909.
Huang X, Li N, Pu Y, Zhang T, Wang B. Neuroprotective effects of ginseng phytochemicals: recent perspectives. Molecules. 2019;24(16):2939.
Moscovich M, LaFaver K, Maetzler W. Functional movement disorder in older adults, in functional movement disorder. Berlin: Springer; 2022. p. 197–203.
Navarro-Lopez EM, Çelikok U, Şengör NS. A dynamical model for the basal ganglia-thalamo-cortical oscillatory activity and its implications in Parkinson’s disease. Cogn Neurodyn. 2021;15(4):693–720.
Callahan JW, Wokosin DL, Bevan MD. Dysregulation of the basal Ganglia indirect pathway in early symptomatic q175 Huntington’s disease mice. J Neurosci. 2022;42(10):2080–102.
Bento-Pereira C, Dinkova-Kostova AT. Activation of transcription factor Nrf2 to counteract mitochondrial dysfunction in Parkinson’s disease. Med Res Rev. 2021;41(2):785–802.
Albert K, Raymundo DP, Panhelainen A, Eesmaa A, Shvachiy L, Araújo GR, et al. Cerebral dopamine neurotrophic factor reduces α-synuclein aggregation and propagation and alleviates behavioral alterations in vivo. Mol Ther. 2021;29(9):2821–40.
Jayaram S, Krishnamurthy PT. Role of microgliosis, oxidative stress and associated neuroinflammation in the pathogenesis of parkinson’s disease: the therapeutic role of Nrf2 activators. Neurochem Int. 2021;145: 105014.
Angelopoulou E, Paudel YN, Julian T, Shaikh MF, Piperi C. Pivotal role of Fyn kinase in Parkinson’s disease and levodopa-induced dyskinesia: a novel therapeutic target? Mol Neurobiol. 2021;58(4):1372–91.
Claassen DO, Ayyagari R, Garcia-Horton V, Zhang S, Alexander J, Leo S. Real-world adherence to tetrabenazine or deutetrabenazine among patients with huntington’s disease: a retrospective database analysis. Neurology and Therapy. 2022;11(1):435–48.
Sivanandy P, Leey TC, **ang TC, Ling TC, Wey Han SA, Semilan SLA, et al. Systemic review on parkinson’s disease medications, emphasizing on three recently approved drugs to control Parkinson’s symptoms. Int J Environ Res Public Health. 2022;19(1):364.
Avcı B, Günaydın C, Güvenç T, Yavuz CK, Kuruca N, Bilge SS. Idebenone ameliorates rotenone-induced Parkinson’s disease in rats through decreasing lipid peroxidation. Neurochem Res. 2021;46(3):513–22.
Tabrizi SJ, Flower MD, Ross CA, Wild EJ. Huntington disease: new insights into molecular pathogenesis and therapeutic opportunities. Nat Rev Neurol. 2020;16(10):529–46.
Hua K-F, Chao A-C, Lin T-Y, Chen W-T, Lee Y-C, Hsu W-H, et al. Ginsenoside compound K reduces the progression of Huntington’s disease via the inhibition of oxidative stress and overactivation of the Atm/Ampk pathway. J Ginseng Res. 2021;2:2.
Crowell V, Houghton R, Tomar A, Fernandes T, Squitieri F. Modeling manifest Huntington’s disease prevalence using diagnosed incidence and survival time. Neuroepidemiology. 2021;55(5):361–8.
Maity S, Komal P, Kumar V, Saxena A, Tungekar A, Chandrasekar V. Impact of Er stress and Er-mitochondrial crosstalk in Huntington’s disease. Int J Mol Sci. 2022;23(2):780.
Deepa S, Rymbai E, Praveen T, Saravanan J. Neuroprotective effects of farnesol on motor and cognitive impairment against 3-nitropropionic acid-induced Huntington’s disease. Thai J Pharm Sci. 2021;45:1.
Naia L, Carmo C, Campesan S, Fao L, Cotton VE, Valero J, et al. Mitochondrial Sirt3 confers neuroprotection in Huntington’s disease by regulation of oxidative challenges and mitochondrial dynamics. Free Radical Biol Med. 2021;163:163–79.
Hu X, Zhang D, Pang H, Caudle WM, Li Y, Gao H, et al. Macrophage antigen complex-1 mediates reactive microgliosis and progressive dopaminergic neurodegeneration in the Mptp model of Parkinson’s disease. J Immunol. 2008;181(10):7194–204.
Kormas P, Moutzouri A. Current psychological approaches in neurodegenerative diseases, in handbook of computational neurodegeneration. Berlin: Springer; 2022. p. 1–29.
Dinkova-Kostova AT, Abramov AY. The emerging role of Nrf2 in mitochondrial function. Free Radical Biol Med. 2015;88:179–88.
Zeng X-H, Li Q-Q, Xu Q, Li F, Liu C-Z. Acupuncture mechanism and redox equilibrium. Evid-Based Complement Alternat Med. 2014;2:2.
Huang T-I, Hsieh C-L. Effects of acupuncture on oxidative stress amelioration via Nrf2/are-related pathways in Alzheimer and Parkinson diseases. Evid-Based Complement Alternat Med. 2021;2:2.
Zhu J, Xu X, Liang Y, Zhu R. Downregulation of microrna-15b-5p targeting the Akt3-mediated Gsk-3β/Β-catenin signaling pathway inhibits cell apoptosis in Parkinson’s disease. BioMed Res Int. 2021;2:2.
Pederson BA. Structure and regulation of glycogen synthase in the brain. Brain Glycogen Metab. 2019;2:83–123.
Patel P, Woodgett JR. Glycogen synthase kinase 3: a kinase for all pathways? Curr Top Dev Biol. 2017;123:277–302.
Li J, Ma S, Chen J, Hu K, Li Y, Zhang Z, et al. Gsk-3β contributes to parkinsonian dopaminergic neuron death: evidence from conditional knockout mice and tideglusib. Front Mol Neurosci. 2020;13:81.
Juhaszova M, Zorov DB, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Role of glycogen synthase kinase-3β in cardioprotection. Circ Res. 2009;104(11):1240–52.
Valvezan AJ, Klein PS. Gsk-3 and Wnt signaling in neurogenesis and bipolar disorder. Front Mol Neurosci. 2012;5:1.
Golpich M, Amini E, Hemmati F, Ibrahim NM, Rahmani B, Mohamed Z, et al. Glycogen synthase kinase-3 beta (Gsk-3β) signaling: implications for Parkinson’s disease. Pharmacol Res. 2015;97:16–26.
Roca C, Campillo NE. Glycogen synthase kinase 3 (Gsk-3) inhibitors: a patent update (2016–2019). Expert Opin Ther Pat. 2020;30(11):863–72.
Zarneshan SN, Fakhri S, Khan H. Targeting Akt/Creb/Bdnf signaling pathway by ginsenosides in neurodegenerative diseases: a mechanistic approach. Pharmacol Res. 2022;2:106099.
Sharma N, Khurana N, Muthuraman A, Utreja P. Pharmacological evaluation of vanillic acid in rotenone-induced Parkinson’s disease rat model. Eur J Pharmacol. 2021;903: 174112.
Buyan-Dent L, Mangin T, Shannon KM. Pharmaceutical treatment of Parkinson’s disease. Pract Neurol. 2018;2:2.
Morgante F, Bavikatte G, Anwar F, Mohamed B. The burden of sialorrhoea in chronic neurological conditions: current treatment options and the role of incobotulinumtoxina (Xeomin®). Ther Adv Neurol Disord. 2019;12:1756286419888601.
Yamaguchi A, Ishikawa K-i, Inoshita T, Shiba-Fukushima K, Saiki S, Hatano T, et al. Identifying therapeutic agents for amelioration of mitochondrial clearance disorder in neurons of familial Parkinson disease. Stem Cell Rep. 2020;14(6):1060–75.
Long H-Z, Cheng Y, Zhou Z-W, Luo H-Y, Wen D-D, Gao L-C. Pi3k/Akt signal pathway: a target of natural products in the prevention and treatment of Alzheimer’s disease and Parkinson’s disease. Front Pharmacol. 2021;12:619.
Zhang L, Cen L, Qu S, Wei L, Mo M, Feng J, et al. Enhancing beta-catenin activity via Gsk3beta inhibition protects Pc12 cells against rotenone toxicity through Nurr1 induction. PLoS ONE. 2016;11(4): e0152931.
Wang W, Yang Y, Ying C, Li W, Ruan H, Zhu X, et al. Inhibition of glycogen synthase kinase-3β protects dopaminergic neurons from Mptp toxicity. Neuropharmacology. 2007;52(8):1678–84.
Morales-García JA, Susín C, Alonso-Gil S, Pérez DI, Palomo V, Pérez C, et al. Glycogen synthase kinase-3 inhibitors as potent therapeutic agents for the treatment of Parkinson disease. ACS Chem Neurosci. 2013;4(2):350–60.
Chen G, Bower KA, Ma C, Fang S, Thiele CJ, Luo J. Glycogen synthase kinase 3β (Gsk3β) mediates 6-hydroxydopamine-induced neuronal death. FASEB J. 2004;18(10):1162–4.
Cuadrado A, Moreno-Murciano P, Pedraza-Chaverri J. The transcription factor Nrf2 as a new therapeutic target in Parkinson’s disease. Expert Opin Ther Targets. 2009;13(3):319–29.
Clark J, Simon DK. Transcribe to survive: transcriptional control of antioxidant defense programs for neuroprotection in Parkinson’s disease. Antioxid Redox Signal. 2009;11(3):509–28.
Valencia A, Reeves PB, Sapp E, Li X, Alexander J, Kegel KB, et al. Mutant huntingtin and glycogen synthase kinase 3-Β accumulate in neuronal lipid rafts of a presymptomatic knock-in mouse model of Huntington’s disease. J Neurosci Res. 2010;88(1):179–90.
Carmichael J, Sugars KL, Bao YP, Rubinsztein DC. Glycogen synthase kinase-3β inhibitors prevent cellular polyglutamine toxicity caused by the huntington’s disease mutation. J Biol Chem. 2002;277(37):33791–8.
Ruiz SMA, Eldar-Finkelman H. Glycogen synthase kinase-3 inhibitors: preclinical and clinical focus on Cns-a decade onward. Front Mol Neurosci. 2021;2:14.
L’episcopo F, Drouin-Ouellet J, Tirolo C, Pulvirenti A, Giugno R, Testa N, et al. Gsk-3β-Induced tau pathology drives hippocampal neuronal cell death in huntington’s disease: involvement of astrocyte-neuron interactions. Cell Death Dis. 2016;7(4):e2206–e2206.
Fernández-Nogales M, Hernández F, Miguez A, Alberch J, Ginés S, Pérez-Navarro E, et al. Decreased glycogen synthase kinase-3 levels and activity contribute to Huntington’s disease. Hum Mol Genet. 2015;24(17):5040–52.
Rippin I, Bonder K, Joseph S, Sarsor A, Vaks L, Eldar-Finkelman H. Inhibition of Gsk-3 ameliorates the pathogenesis of Huntington’s disease. Neurobiol Dis. 2021;154: 105336.
Milutinović A, Zorc-Pleskovič R. Glycogen accumulation in cardiomyocytes and cardiotoxic effects after 3npa treatment. Bosn J Basic Med Sci. 2012;12(1):15.
Wu A-G, Yong Y-Y, Pan Y-R, Zhang L, Wu J-M, Zhang Y, et al. Targeting Nrf2-mediated oxidative stress response in traumatic brain injury: therapeutic perspectives of phytochemicals. Oxid Med Cell Longev. 2022;2:2.
Choi JW, Kim S, Yoo JS, Kim HJ, Kim HJ, Kim BE, et al. Development and optimization of halogenated vinyl sulfones as Nrf2 activators for the treatment of Parkinson’s disease. Eur J Med Chem. 2021;212: 113103.
Scuderi SA, Ardizzone A, Paterniti I, Esposito E, Campolo M. Antioxidant and anti-inflammatory effect of Nrf2 inducer dimethyl fumarate in neurodegenerative diseases. Antioxidants. 2020;9(7):630.
Ayuso P, Martínez C, Pastor P, Lorenzo-Betancor O, Luengo A, Jiménez-Jiménez FJ, et al. An association study between heme oxygenase-1 genetic variants and Parkinson’s disease. Front Cell Neurosci. 2014;8:298.
Nitti M, Piras S, Brondolo L, Marinari UM, Pronzato MA, Furfaro AL. Heme oxygenase 1 in the nervous system: does it favor neuronal cell survival or induce neurodegeneration? Int J Mol Sci. 2018;19(8):2260.
Kumar H, Koppula S, Kim I-S, Vasant More S, Kim B-W, Choi D-K. Nuclear Factor erythroid 2-related factor 2 signaling in parkinson disease: a promising multi therapeutic target against oxidative stress, neuroinflammation and cell death. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders). 2012; 11(8): 1015–1029.
Alonso-Navarro H, Jiménez-Jiménez F, Pilo de la Fuente B, and Plaza-Nieto J. Mecanismos Patogénicos De La Enfermedad De Parkinson. Tratado de los Trastornos del Movimiento. 2nd edition (ISBN 978–84–85424–76–4). 2008: 425–485.
Hochstrasser H, Bauer P, Walter U, Behnke S, Spiegel J, Csoti I, et al. Ceruloplasmin gene variations and substantia nigra hyperechogenicity in parkinson disease. Neurology. 2004;63(10):1912–7.
Sofic E, Riederer P, Heinsen H, Beckmann H, Reynolds G, Hebenstreit G, et al. Increased iron (Iii) and total iron content in post mortem substantia nigra of parkinsonian brain. J Neural Transm. 1988;74(3):199–205.
Urrutia P, Bórquez D, Núñez M. Inflaming the brain with iron. Antioxidants. 2021;10:61.
Weinreb O, Mandel S, Youdim MB, Amit T. Targeting dysregulation of brain iron homeostasis in parkinson’s disease by iron chelators. Free Radical Biol Med. 2013;62:52–64.
Vašková J, Vaško L, Kron I. Oxidative processes and antioxidative metaloenzymes. Antioxid Enzyme. 2012;2:19–58.
Garza-Lombó C, Franco R. The environmental contribution to redox dyshomeostasis in parkinson’s disease, in parkinsonism and the environment. Berlin: Springer; 2022. p. 69–102.
Ito H, Kurokawa H, Matsui H. Mitochondrial reactive oxygen species and heme, non-heme iron metabolism. Arch Biochem Biophys. 2021;700: 108695.
He Q, Song N, Jia F, Xu H, Yu X, **e J, et al. Role of Α-synuclein aggregation and the nuclear factor E2-related factor 2/Heme oxygenase-1 pathway in iron-induced neurotoxicity. Int J Biochem Cell Biol. 2013;45(6):1019–30.
Delaidelli A, Richner M, Jiang L, van der Laan A, Bergholdt Jul Christiansen I, Ferreira N, et al. Α-synuclein pathology in parkinson disease activates homeostatic Nrf2 anti-oxidant response. Acta Neuropathol Commun. 2021;9(1):1–16.
Tong H, Zhang X, Meng X, Lu L, Mai D, Qu S. Simvastatin inhibits activation of Nadph oxidase/P38 Mapk pathway and enhances expression of antioxidant protein in parkinson disease models. Front Mol Neurosci. 2018;11:165.
Masaki Y, Izumi Y, Matsumura A, Akaike A, Kume T. Protective effect of Nrf2–are activator isolated from green perilla leaves on dopaminergic neuronal loss in a parkinson’s disease model. Eur J Pharmacol. 2017;798:26–34.
He J, Zhu G, Wang G, Zhang F. Oxidative stress and neuroinflammation potentiate each other to promote progression of dopamine neurodegeneration. Oxid Med Cell Longev. 2020;2:2.
Min K-J, Yang M-s, Kim S-U, Jou I, Joe E-H. Astrocytes induce hemeoxygenase-1 expression in microglia: a feasible mechanism for preventing excessive brain inflammation. J Neurosci. 2006;26(6):1880–7.
Rojo AI, Innamorato NG, Martín-Moreno AM, De Ceballos ML, Yamamoto M, Cuadrado A. Nrf2 regulates microglial dynamics and neuroinflammation in experimental Parkinson’s disease. Glia. 2010;58(5):588–98.
Izumi Y, Kataoka H, Inose Y, Akaike A, Koyama Y, Kume T. Neuroprotective effect of an Nrf2-are activator identified from a chemical library on dopaminergic neurons. Eur J Pharmacol. 2018;818:470–9.
Upadhayay S, Mehan S. Targeting Nrf2/Ho-1 anti-oxidant signaling pathway in the progression of multiple sclerosis and influences on neurological dysfunctions. Brain Disord. 2021;3: 100019.
Zheng J, Winderickx J, Franssens V, Liu B. A mitochondria-associated oxidative stress perspective on Huntington’s disease. Front Mol Neurosci. 2018;11:329.
Weidinger A, Kozlov AV. Biological activities of reactive oxygen and nitrogen species: oxidative stress versus signal transduction. Biomolecules. 2015;5(2):472–84.
Yang J-L, Weissman L, Bohr VA, Mattson MP. Mitochondrial DNA damage and repair in neurodegenerative disorders. DNA Repair. 2008;7(7):1110–20.
Siddiqui A, Rivera-Sánchez S, Castro MdR, Acevedo-Torres K, Rane A, Torres-Ramos CA, et al. Mitochondrial DNA damage is associated with reduced mitochondrial bioenergetics in Huntington’s disease. Free Radical Biol Med. 2012;53(7):1478–88.
Gao Y, Chu S-F, Li J-P, Zhang Z, Yan J-Q, Wen Z-L, et al. Protopanaxtriol protects against 3-nitropropionic acid-induced oxidative stress in a rat model of huntington’s disease. Acta Pharmacol Sin. 2015;36(3):311–22.
Calkins MJ, Jakel RJ, Johnson DA, Chan K, Kan YW, Johnson JA. Protection from mitochondrial complex ii inhibition in vitro and in vivo by Nrf2-mediated transcription. Proc Natl Acad Sci. 2005;102(1):244–9.
Linseman DA. Targeting oxidative stress for neuroprotection. Antioxid Redox Signal. 2009;11(3):421–4.
Gopinath K, Sudhandiran G. Naringin modulates oxidative stress and inflammation in 3-nitropropionic acid-induced neurodegeneration through the activation of nuclear factor-erythroid 2-related factor-2 signalling pathway. Neuroscience. 2012;227:134–43.
Gonchar OO, Maznychenko AV, Klyuchko OM, Mankovska IM, Butowska K, Borowik A, et al. C60 fullerene reduces 3-nitropropionic acid-induced oxidative stress disorders and mitochondrial dysfunction in rats by modulation of P53, Bcl-2 and Nrf2 targeted proteins. Int J Mol Sci. 2021;22(11):5444.
Nguyen T, Yang CS, Pickett CB. The pathways and molecular mechanisms regulating Nrf2 activation in response to chemical stress. Free Radical Biol Med. 2004;37(4):433–41.
Ikram M, Ullah R, Khan A, Kim MO. Ongoing research on the role of gintonin in the management of neurodegenerative disorders. Cells. 2020;9(6):1464.
Liu N, Bai L, Lu Z, Gu R, Zhao D, Yan F, et al. Trpv4 contributes to Er stress and inflammation: implications for Parkinson’s disease. J Neuroinflammation. 2022;19(1):1–15.
Kim GH, Kim JE, Rhie SJ, Yoon S. The role of oxidative stress in neurodegenerative diseases. Exp Neurobiol. 2015;24(4):325.
Pajares M, Cuadrado A, Rojo AI. Modulation of proteostasis by transcription factor Nrf2 and impact in neurodegenerative diseases. Redox Biol. 2017;11:543–53.
Cores Á, Piquero M, Villacampa M, León R, Menéndez JC. Nrf2 regulation processes as a source of potential drug targets against neurodegenerative diseases. Biomolecules. 2020;10(6):904.
Canning P, Sorrell FJ, Bullock AN. Structural basis of Keap1 interactions with Nrf2. Free Radical Biol Med. 2015;88:101–7.
Rojo AI, Sagarra MR, Cuadrado A. Gsk-3β down-regulates the transcription factor Nrf2 after oxidant damage: relevance to exposure of neuronal cells to oxidative stress. J Neurochem. 2008;105(1):192–202.
Su J, Zhang J, Bao R, **a C, Zhang Y, Zhu Z, et al. Mitochondrial dysfunction and apoptosis are attenuated through activation of Ampk/Gsk-3β/Pp2a pathway in Parkinson’s disease. Eur J Pharmacol. 2021;907: 174202.
Chen X, Liu Y, Zhu J, Lei S, Dong Y, Li L, et al. Gsk-3β downregulates Nrf2 in cultured cortical neurons and in a rat model of cerebral ischemia-reperfusion. Sci Rep. 2016;6(1):1–16.
Han R, Yu Y, Zhao K, Wei J, Hui Y, Gao J-M. Lignans from eucommia ulmoides oliver leaves exhibit neuroprotective effects via activation of the Pi3k/Akt/Gsk-3β/Nrf2 signalling pathways in H2o2-treated Pc-12 cells. Phytomedicine. 2022;2:154124.
Mittal SP, Khole S, Jagadish N, Ghosh D, Gadgil V, Sinkar V, et al. Andrographolide protects liver cells from H2o2 induced cell death by upregulation of Nrf-2/Ho-1 mediated via adenosine A2a receptor signalling. Biochim Biophys Acta General Subj. 2016;1860(11):2377–90.
Habtemariam S. The Nrf2/Ho-1 axis as targets for flavanones: neuroprotection by pinocembrin, naringenin, and eriodictyol. Oxid Med Cell Longev. 2019;2:2.
Armagan G, Sevgili E, Gürkan FT, Köse FA, Bilgiç T, Dagcı T, et al. Regulation of the Nrf2 pathway by glycogen synthase kinase-3β in Mpp+-induced cell damage. Molecules. 2019;24(7):1377.
Salazar M, Rojo AI, Velasco D, de Sagarra RM, Cuadrado A. Glycogen synthase kinase-3β inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor Nrf2. J Biol Chem. 2006;281(21):14841–51.
Zhang X-L, Yuan Y-H, Shao Q-H, Wang Z-Z, Zhu C-G, Shi J-G, et al. Dj-1 regulating Pi3k-Nrf2 signaling plays a significant role in bibenzyl compound 20c-mediated neuroprotection against rotenone-induced oxidative insult. Toxicol Lett. 2017;271:74–83.
Khan A, Jamwal S, Bijjem K, Prakash A, Kumar P. Neuroprotective effect of hemeoxygenase-1/glycogen synthase kinase-3β modulators in 3-nitropropionic acid-induced neurotoxicity in rats. Neuroscience. 2015;287:66–77.
Infante J, García-Gorostiaga I, Sánchez-Juan P, Sierra M, Martín-Gurpegui J, Terrazas J, et al. Synergistic effect of two oxidative stress-related genes (heme oxygenase-1 and Gsk3β) on the risk of parkinson’s disease. Eur J Neurol. 2010;17(5):760–2.
Arab HH, Safar MM, Shahin NN. Targeting ros-dependent Akt/Gsk-3β/Nf-Κb and Dj-1/Nrf2 pathways by dapagliflozin attenuates neuronal injury and motor dysfunction in rotenone-induced parkinson’s disease rat model. ACS Chem Neurosci. 2021;12(4):689–703.
Huang Y, Sun L, Zhu S, Xu L, Liu S, Yuan C, et al. Neuroprotection against Parkinson’s disease through the activation of Akt/Gsk3β signaling pathway by tovophyllin A. Front Neurosci. 2020;14:723.
Wu C-R, Chang H-C, Cheng Y-D, Lan W-C, Yang S-E, Ching H. Aqueous extract of davallia mariesii attenuates 6-hydroxydopamine-induced oxidative damage and apoptosis in B35 cells through inhibition of caspase cascade and activation of Pi3k/Akt/Gsk-3β pathway. Nutrients. 2018;10(10):1449.
Nataraj J, Manivasagam T, Justin Thenmozhi A, Essa MM. Neurotrophic effect of asiatic acid, a triterpene of centella asiatica against chronic 1-methyl 4-phenyl 1, 2, 3, 6-tetrahydropyridine hydrochloride/probenecid mouse model of Parkinson’s disease: the role of Mapk, Pi3k-Akt-Gsk3β and Mtor signalling pathways. Neurochem Res. 2017;42(5):1354–65.
Wang H-M, Zhang T, Li Q, Huang J-K, Chen R-F, Sun X-J. Inhibition of glycogen synthase kinase-3β by lithium chloride suppresses 6-hydroxydopamine-induced inflammatory response in primary cultured astrocytes. Neurochem Int. 2013;63(5):345–53.
Duka T, Duka V, Joyce JN, Sidhu A. Α-synuclein contributes to Gsk-3β-catalyzed tau phosphorylation in Parkinson’s disease models. FASEB J. 2009;23(9):2820–30.
Inose Y, Izumi Y, Takada-Takatori Y, Akaike A, Koyama Y, Kaneko S, et al. Protective effects of Nrf2–are activator on dopaminergic neuronal loss in parkinson disease model mice: possible involvement of heme oxygenase-1. Neurosci Lett. 2020;736: 135268.
Darabi S, Noori-Zadeh A, Abbaszadeh H-A, Rajaei F, Bakhtiyari S. Trehalose neuroprotective effects on the substantia nigra dopaminergic cells by activating autophagy and non-canonical Nrf2 pathways. Iran J Pharmac Res IJPR. 2019;18(3):1419.
Moreira S, Fonseca I, Nunes MJ, Rosa A, Lemos L, Rodrigues E, et al. Nrf2 activation by tauroursodeoxycholic acid in experimental models of Parkinson’s disease. Exp Neurol. 2017;295:77–87.
Michel HE, Tadros MG, Esmat A, Khalifa AE, Abdel-Tawab AM. Tetramethylpyrazine ameliorates rotenone-induced parkinson’s disease in rats: involvement of its anti-inflammatory and anti-apoptotic actions. Mol Neurobiol. 2017;54(7):4866–78.
Cui Q, Li X, Zhu H. Curcumin ameliorates dopaminergic neuronal oxidative damage via activation of the Akt/Nrf2 pathway. Mol Med Rep. 2016;13(2):1381–8.
Innamorato NG, Jazwa A, Rojo AI, García C, Fernández-Ruiz J, Grochot-Przeczek A, et al. Different susceptibility to the Parkinson’s toxin mptp in mice lacking the redox master regulator Nrf2 or its target gene heme oxygenase-1. PLoS ONE. 2010;5(7): e11838.
Salinas M, Diaz R, Abraham NG, de Galarreta CMR, Cuadrado A. Nerve growth factor protects against 6-hydroxydopamine-induced oxidative stress by increasing expression of heme oxygenase-1 in a phosphatidylinositol 3-kinase-dependent manner. J Biol Chem. 2003;278(16):13898–904.
Silva-Palacios A, Ostolga-Chavarría M, Buelna-Chontal M, Garibay C, Hernández-Reséndiz S, Roldán F, et al. 3-Np-induced Huntington’s-like disease impairs Nrf2 activation without loss of cardiac function in aged rats. Exp Gerontol. 2017;96:89–98.
Liu P, Li Y, Yang W, Liu D, Ji X, Chi T, et al. Prevention of Huntington’s disease-like behavioral deficits in R6/1 mouse by tolfenamic acid is associated with decreases in mutant huntingtin and oxidative stress. Oxid Med Cell Longev. 2019;2:2.
Shih AY, Imbeault S, Barakauskas V, Erb H, Jiang L, Li P, et al. Induction of the Nrf2-driven antioxidant response confers neuroprotection during mitochondrial stress in vivo. J Biol Chem. 2005;280(24):22925–36.
Acknowledgements
Authors would like to sincerely acknowledge Department of Science & Technology (DST), New Delhi for providing INSPIRE fellowship to Divya Soni.
Funding
This review did not receive any specific grant from funding agencies,
Author information
Authors and Affiliations
Contributions
DS: data acquisition, concept design, and drafting manuscript. PK: concept design, supervision, edit and final approval of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Soni, D., Kumar, P. GSK-3β-mediated regulation of Nrf2/HO-1 signaling as a new therapeutic approach in the treatment of movement disorders. Pharmacol. Rep 74, 557–569 (2022). https://doi.org/10.1007/s43440-022-00390-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-022-00390-z