Abstract
Background
Recruitment of β-arrestin to G protein-coupled receptors (GPCRs), initially described to cause receptor desensitization, has recently been shown to take active roles in cell signaling. We investigated the effects of TRV027, an angiotensin AT1 receptor β-arrestin-biased ligand, as well as losartan and valsartan on cisplatin-induced kidney injury.
Method
Male Sprague–Dawley rats were treated with angiotensin receptor ligands (1 or 10 mg/kg/day) with or without cisplatin, and kidney variables were monitored using animal SPECT, histopathology, and serum parameters.
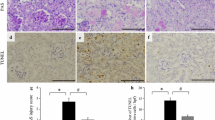
Results
TRV027, losartan, and valsartan did not alter renal dimercaptosuccinic acid (DMSA) uptake, histopathological manifestations of kidney injury, blood urea nitrogen (BUN), and creatinine or Na+ and K+ levels, per se. However, when rats co-treated with cisplatin and either of the AT1 receptor blockers at higher doses, we observed aggravation of cisplatin-induced reduction of radiotracer uptake but improvement of cisplatin-induced hypokalemia, and insignificant effect on histological findings. Furthermore, we noted an additional increase in cisplatin-induced augmentation of BUN and creatinine levels in cisplatin plus valsartan group. TRV027 (1 mg/kg/day) inhibited cisplatin adverse effects on radiotracer uptake, kidney histology, BUN, and creatinine as well as electrolyte levels, but it failed to produce protective effects at higher dose (10 mg/kg/day).
Conclusion
Low-dose TRV027 may offer potential benefits in kidney injury due to cisplatin.




Similar content being viewed by others
References
dos Santos NA, Carvalho Rodrigues MA, Martins NM, dos Santos AC. Cisplatin-induced nephrotoxicity and targets of nephroprotection: an update. Arch Toxicol [Internet]. 2012;86(8):1233–50.
Yuwen D, Mi S, Ma Y, Guo W, Xu Q, Shen Y, Shu Y. Andrographolide enhances cisplatin-mediated anticancer effects in lung cancer cells through blockade of autophagy. Anticancer Drugs [Internet]. 2017;28(9):967–76.
Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int [Internet]. 2008;73(9):994–1007.
Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am J Med Sci. 2007;334(2):115–24.
Wzgarda A, Kleszcz R, Prokop M, Regulska K, Regulski M, Paluszczak J, et al. Unknown face of known drugs - what else can we expect from angiotensin converting enzyme inhibitors? Eur J Pharmacol [Internet]. 2017;797:9–19.
Johnson SA, Spurney RF. Twenty years after ACEIs and ARBs: emerging treatment strategies for diabetic nephropathy. Am J Physiol Ren Physiol [Internet]. 2015;309(10):F807–20.
Padi SSV, Chopra K. Selective angiotensin II type 1 receptor blockade ameliorates cyclosporine nephrotoxicity. Pharmacol Res [Internet]. 2002;45(5):413–20.
Anarat A, Noyan A, Gonlusen G, Duman N, Tuncer D. Influence of enalapril on experimental cyclosporin A nephrotoxicity. Pediatr Nephrol [Internet]. 1996;10(5):616–20.
el El-Sayed SM, Abd-Ellah MF, Attia SM. Protective effect of captopril against cisplatin-induced nephrotoxicity in rats. Pak J Pharm Sci. 2008;21(3):255–61.
Saleh S, Ain-Shoka AA, El-Demerdash E, Khalef MM. Protective effects of the angiotensin II receptor blocker losartan on cisplatin-induced kidney injury. Chemotherapy [Internet]. 2009;55(6):399–406.
Haghighi M, Nematbakhsh M, Talebi A, Nasri H, Ashrafi F, Roshanaei K, et al. The role of angiotensin II receptor 1 (AT1) blockade in cisplatin-induced nephrotoxicity in rats: gender-related differences. Ren Fail [Internet]. 2012;34(8):1046–51.
Zamani Z, Nematbakhsh M, Eshraghi-Jazi F, Talebi A, Jilanchi S, Navidi M, et al. Effect of enalapril in cisplatin-induced nephrotoxicity in rats; gender-related difference. Adv Biomed Res [Internet]. 2016;5:14.
Goldstein SL, Mottes T, Simpson K, Barclay C, Muething S, Haslam DB, et al. A sustained quality improvement program reduces nephrotoxic medication-associated acute kidney injury. Kidney Int [Internet]. 2016;90(1):212–21.
Spiotto MT, Cao H, Mell L, Toback FG. Angiotensin-converting enzyme inhibitors predict acute kidney injury during chemoradiation for head and neck cancer. Anticancer Drugs [Internet]. 2015;26(3):343–9.
Almanric K, Marceau N, Cantin A, Bertin É. Risk factors for nephrotoxicity associated with cisplatin. Can J Hosp Pharm [Internet]. 2017;70(2):99–106.
Seyedabadi M, Ghahremani MH, Albert PR. Biased signaling of G protein coupled receptors (GPCRs): molecular determinants of GPCR/transducer selectivity and therapeutic potential. Pharmacol Ther [Internet]. 2019;200:148–78.
Seyedabadi M, Ostad SN, Albert PR, Dehpour AR, Rahimian R, Ghazi-Khansari M, et al. Ser/ Thr residues at α3/β5 loop of Gαs are important in morphine-induced adenylyl cyclase sensitization but not mitogen-activated protein kinase phosphorylation. FEBS J [Internet]. 2012;279(4):650–60.
Park JH, Scheerer P, Hofmann KP, Choe H-W, Ernst OP. Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature [Internet]. 2008;454(7201):183–7.
Lymperopoulos A, Aukszi B. Angiotensin receptor blocker drugs and inhibition of adrenal beta-arrestin-1-dependent aldosterone production: implications for heart failure therapy. World J Cardiol [Internet]. 2017;9(3):200.
Dabul S, Bathgate-Siryk A, Valero TR, Jafferjee M, Sturchler E, McDonald P, et al. Suppression of adrenal βarrestin1-dependent aldosterone production by ARBs: head-to-head comparison. Sci Rep [Internet]. 2015;5:8116.
Violin JD, DeWire SM, Yamashita D, Rominger DH, Nguyen L, Schiller K, et al. Selectively engaging β-arrestins at the angiotensin II type 1 receptor reduces blood pressure and increases cardiac performance. J Pharmacol Exp Ther [Internet]. 2010;335(3):572–9.
Fatemikia H, Seyedabadi M, Karimi Z, Tanha K, Assadi M, Tanha K. Comparison of 99mTc-DMSA renal scintigraphy with biochemical and histopathological findings in animal models of acute kidney injury. Mol Cell Biochem [Internet]. 2017;434(1–2):163–9.
Tanha K, Fatemikia H, Assadi M, Seyedabadi M. Assessment of the maximum uptake time of 99mTc-DMSA in renal scintigraphy in rat. Iran J Nucl Med [Internet]. 2017;25(2):110–4.
Wellington D, Mikaelian I, Singer L. Comparison of ketamine-xylazine and ketamine-dexmedetomidine anesthesia and intraperitoneal tolerance in rats. J Am Assoc Lab Anim Sci. 2013;52(4):481–7.
Ishida S, Lee J, Thiele DJ, Herskowitz I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci USA. 2002;99(22):14298–302.
Hanigan MH, Devarajan P. Cisplatin nephrotoxicity: molecular mechanisms. Cancer Ther [Internet]. 2003;1:47–61.
Hye Khan MA, Abdul Sattar M, Abdullah NA, Johns EJ. Cisplatin-induced nephrotoxicity causes altered renal hemodynamics in Wistar Kyoto and spontaneously hypertensive rats: role of augmented renal alpha-adrenergic responsiveness. Exp Toxicol Pathol. 2007;59(3–4):253–60.
Komaki K, Kusaba T, Tanaka M, Kado H, Shiotsu Y, Matsui M, et al. Lower blood pressure and risk of cisplatin nephrotoxicity: a retrospective cohort study. BMC Cancer. 2017;17(1):144.
Mizuno T, Hayashi T, Shimabukuro Y, Murase M, Hayashi H, Ishikawa K, et al. Lower blood pressure-induced renal hypoperfusion promotes cisplatin-induced nephrotoxicity. Oncology. 2016;90(6):313–20.
Peters AM, Jones DH, Evans K, Gordon I. Two routes for 99mTc-DMSA uptake into the renal cortical tubular cell. Eur J Nucl Med. 1988;14(11):555–61.
Criscione L, de Gasparo M, Buhlmayer P, Whitebread S, Ramjoue HP, Wood J. Pharmacological profile of valsartan: a potent, orally active, nonpeptide antagonist of the angiotensin II AT1-receptor subtype. Br J Pharmacol. 1993;110(2):761–71.
Le MT, Pugsley MK, Vauquelin G, Van Liefde I. Molecular characterisation of the interactions between olmesartan and telmisartan and the human angiotensin II AT(1) receptor. Br J Pharmacol. 2007;151:952–62.
Miura S, Karnik SS, Saku K. Review: angiotensin II type 1 receptor blockers: class effects versus molecular effects. J Renin Angiotensin Aldosterone Syst [Internet]. 2011;12(1):1–7.
Das S, Bandyopadhyay S, Ramasamy A, Prabhu VV, Pachiappan S. A case of losartan-induced severe hyponatremia. J Pharmacol Pharmacother [Internet]. 2015;6(4):219–21.
Miao Y, Dobre D, Heerspink HJL, Brenner BM, Cooper ME, Parving H-H, et al. Increased serum potassium affects renal outcomes: a post hoc analysis of the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial. Diabetologia. 2011;54(1):44–50.
Goldberg MR, Tanaka W, Barchowsky A, Bradstreet TE, McCrea J, Lo MW, et al. Effects of losartan on blood pressure, plasma renin activity, and angiotensin II in volunteers. Hypertens (Dallas, Tex 1979). 1993;21(5):704–13.
Kim D-R, Cho J-H, Jang W-S, Kim J-S, Jeong K-H, Lee T-W, et al. Severe hyponatremia associated with the use of angiotensin II receptor blocker/thiazide combinations. Electrolyte Blood Press E BP. 2013;11:56–9.
Bjorck S, Mulec H, Johnsen SA, Norden G, Aurell M, Björck S, et al. Renal protective effect of enalapril in diabetic nephropathy. BMJ [Internet]. 1992;304(6823):339–43.
Bilan VP, Salah EM, Bastacky S, Jones HB, Mayers RM, Zinker B, et al. Diabetic nephropathy and long-term treatment effects of rosiglitazone and enalapril in obese ZSF1 rats. J Endocrinol. 2011;210(3):293–308.
Ishidoya S, Morrissey J, McCracken R, Klahr S. Delayed treatment with enalapril halts tubulointerstitial fibrosis in rats with obstructive nephropathy. Kidney Int. 1996;49(4):1110–9.
Zafar I, Tao Y, Falk S, McFann K, Schrier RW, Edelstein CL. Effect of statin and angiotensin-converting enzyme inhibition on structural and hemodynamic alterations in autosomal dominant polycystic kidney disease model. Am J Physiol Renal Physiol. 2007;293(3):F854–9.
Kocak C, Kocak FE, Akcilar R, Bayat Z, Aras B, Metineren MH, et al. Effects of captopril, telmisartan and bardoxolone methyl (CDDO-Me) in ischemia-reperfusion-induced acute kidney injury in rats: an experimental comparative study. Clin Exp Pharmacol Physiol. 2016;43(2):230–41.
Mansour MA, El-Kashef HA, Al-Shabanah OA. Effect of captopril on doxorubicin-induced nephrotoxicity in normal rats. Pharmacol Res. 1999;39(3):233–7.
Okui S, Yamamoto H, Li W, Gamachi N, Fujita Y, Kashiwamura S-I, et al. Cisplatin-induced acute renal failure in mice is mediated by chymase-activated angiotensin-aldosterone system and interleukin-18. Eur J Pharmacol [Internet]. 2012;685(1–3):149–55.
Cotter G, Davison BA, Butler J, Collins SP, Ezekowitz JA, Felker GM, et al. Relationship between baseline systolic blood pressure and long-term outcomes in acute heart failure patients treated with TRV027: an exploratory subgroup analysis of BLAST-AHF. Clin Res Cardiol. 2018;107(2):170–81.
Kim K-S, Abraham D, Williams B, Violin JD, Mao L, Rockman HA. beta-Arrestin-biased AT1R stimulation promotes cell survival during acute cardiac injury. Am J Physiol Heart Circ Physiol. 2012;303(8):H1001–10.
Mukhopadhyay P, Baggelaar M, Erdelyi K, Cao Z, Cinar R, Fezza F, et al. The novel, orally available and peripherally restricted selective cannabinoid CB2 receptor agonist LEI-101 prevents cisplatin-induced nephrotoxicity. Br J Pharmacol. 2016;173(3):446–58.
Wang Y, Huang J, Liu X, Niu Y, Zhao L, Yu Y, et al. beta-Arrestin-biased AT1R stimulation promotes extracellular matrix synthesis in renal fibrosis. Am J Physiol Renal Physiol. 2017;313(1):F1-8.
Ó hAinmhire E, Humphreys BD. Fibrotic changes mediating acute kidney injury to chronic kidney disease transition. Nephron [Internet]. 2017;137(4):264–7.
Hultstrom M, Becirovic-Agic M, Jonsson S. Comparison of acute kidney injury of different etiology reveals in-common mechanisms of tissue damage. Physiol Genomics. 2018;50(3):127–41.
Zuk A, Bonventre JV. Acute kidney injury. Ann Rev Med. 2016;67:293–307.
Funding
The study was supported by the deputy of research of Bushehr University of Medical Sciences. We are grateful to Dr. Jonathan Violin for fruitful discussion about pharmacological profile of angiotensin receptor modulators, especially TRV027. We thank Tehrandaru pharmaceutical company for providing losartan and valsartan APIs, and Dr. Kiarash Tanha for his consult on statistical analysis.
Author information
Authors and Affiliations
Contributions
A.E. performed pathological examinations, F.E. performed animal experiments, prepared serum and tissue samples, M.A. and K.T. conducted scintigraphy experiments, and M.S. developed the idea, supervised the experiments, analyzed the data, and wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests with respect to the publication of the manuscript in the current format.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Esmaeeli, A., Ebrahimi, F., Tanha, K. et al. Low-dose angiotensin AT1 receptor β-arrestin-biased ligand, TRV027, protects against cisplatin-induced nephrotoxicity. Pharmacol. Rep 72, 1676–1684 (2020). https://doi.org/10.1007/s43440-020-00172-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-020-00172-5