Abstract
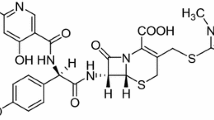
This research focuses explicitly on the solubility of azlocillin and nimodipine in mixed binary solvents. This is relevant for pharmaceutical companies involved in develo** and formulating these drugs. At a constant pressure of 101.2 kPa and temperatures ranging from 283.15 K to 323.15 K, the mole fraction equilibrium solubility of nimodipine and azlocillin in binary solvents (ethanol + ethyl acetate) was determined experimentally by gravimetric method. The results demonstrated that azlocillin and nimodipine solubility improved with higher ethanol mole fractions in mixed solvent systems. Among the three thermodynamic models, the experimental solubility data was best explained by the van't Hoff-Jouyban-Acree (V-J-A) model, followed by the Jouyban-Acree (J-A) model. The greatest RAD and RMSD values occur when two variables are compared to one another (RMSD) were 4.96 × 10−2 and 10.62 × 10−4 for azlocillin and 1.463 × 10−2 and 2.393 × 10−4 for nimodipine, respectively. The preferential solvation parameter \({\delta x}_{1,drug}\) is more significant than zero for nimodipine and azlocillin in the respective ranges of 0.60 < xE < 1 and 0.60 − 0.65 < xE < 1. This indicates that these two studied drug compounds prefer solvated by ethanol over ethyl acetate under these conditions. These results offer valuable perspectives for researchers in the pharmaceutical sciences, especially regarding the comprehension of drug compound solubility and solvation behavior in mixed solvent systems.





Similar content being viewed by others
Data availability
The data that supports the findings of this study are available within the article [and its supplementary material].
References
AlSheyyab RY, Obeidat RM, Altall YR, Abuhuwaij RT, Ghanma RR, Ailabouni AS, Mashaqbeh HA, Al-Haj S (2019) Solubility enhancement of nimodipine through preparation of Soluplus® dispersions. J Appl Pharm Sci 9:30–37
Aulton ME (2002) Pharmaceutics. The science of dosage forms design, second ed. Churchill Livingstone, London
Avdeef A (2003) Absorption and drug development, solubility. In: Permeability and charge State,Wiley-Interscience, Hoboken, NJ
Babu GVMM, Prasad CDS, Murthy KVR (2002) Evaluation of modified gum karaya as carrier for the dissolution enhancement of poorly water-soluble drug nimodipine. Int J Pharm 234:1–17
Cárdenas ZJ, Jiménez DM, Delgado DR, Almanza OA, Jouyban A, Martínez F, Acree WE Jr (2017) Solubility and preferential solvation of some n-alkylparabens in methanol + water mixtures at 298.15 K. J Chem Thermodyn 108:26–37
Cárdenas ZJ, Almanza OA, Jouyban A, Martínez F, Acree WE Jr (2018) Solubility and preferential solvation of phenacetin in methanol + water mixtures at 298.15 K. Phys Chem Liq 56:16–32
Chen GQ, Chen J, Cheng C, Cong Y, Du CB, Zhao HK (2017a) Solubility and preferential solvation of econazole nitrate in binary solvent mixtures of methanol, ethanol and 1,4-dioxane in water. J Chem Thermodyn 111:228–237
Chen J, Chen GQ, Cheng C, Cong Y, Li XH, Zhao HK (2017b) Equilibrium solubility, dissolution thermodynamics and preferential solvation of adenosine in aqueous solutions of N, N dimethylformamide, N-methyl-2-pyrrolidone, dimethylsulfoxide and propylene glycol. J Chem Thermodyn 115:52–62
Chen J, Chen GQ, Cong Y, Du CB, Zhao HK (2017c) Solubility modelling and preferential solvation of paclobutrazol in co-solvent mixtures of (ethanol, n-propanol and 1,4-dioxane) + water. J Chem Thermodyn 112:249–258
Delgado DR, Martínez F (2014) Solubility and preferential solvation of sulfadiazine in methanol + water mixtures at several temperatures. Fluid Phase Equilib 379:128–138
Delgado DR, Peña MÁ, Martínez F, Jouyban A, Acree WE Jr (2016) Further numerical analyses on the solubility of sulfapyridine in ethanol + water mixtures. Pharm Sci 22:143–152
Eghrary SH, Zarghami R, Martinez F, Jouyban A (2012) Solubility of 2-butyl-3- benzofuranyl 4-(2-(diethylamino)ethoxy)-3,5-diiodophenyl ketone hydrochloride (amiodarone HCl) in ethanol + water and n-methyl-2- pyrrolidone + water mixtures at various temperatures. J Chem Eng Data 57:1544–1550
Fedors RF (1974) A method for estimating both the solubility parameters and molar volumes of liquids. Polym Eng Sci 14:147–154
Jabbari M, Khosravi N, Feizabadi M, Ajloo D (2017) Solubility temperature and solvent dependence and preferential solvation of citrus flavonoid naringin in aqueous DMSO mixtures: an experimental and molecular dynamics simulation study. RSC Adv 7:14776–14789
Jouyban A (2010) Handbook of solubility data for pharmaceuticals. CRC Press, Boca Raton
Jouyban A (2008) Review of the cosolvency models for predicting solubility of drugs in water-cosolvent mixtures. J Pharm Pharm Sci 11:32–58
Jouyban A, Fakhree MAA, Acree WE Jr (2012) Comment on Measurement and correlation of solubilities of (z)-2-(2-aminothiazol-4-yl)-2-methoxyiminoacetic acid in different pure solvents and binary mixtures of water + (ethanol, methanol, or glycol). J Chem Eng Data 57:1344–1346
Jouyban A, Acree WE Jr, Martínez F (2016) Modelling the solubility and preferential solvation of gallic acid in cosolvent + Water Mixtures. J Mol Liq 224:502–506
Koenig HB, Metzer KG, Offe HA, Schroeck W (1982) Azlocillin. Ein neues penicillinaus der acylureidoreihe. Synthese und chemischeeigenschaften. Eur J Med Chem 17:59–63
Li X, Ma M, Chen J, Chen G, Zhao H (2017) Preferential solvation of boscalid in ethanol / isopropanol + ethyl acetate mixtures from the inverse kirkwood-buff integrals method. J Solution Chem 46:2050–2065
Marcus Y (2002) Solvent mixtures: properties and selective solvation. Marcel Dekker, New York
Marcus Y (2013) Preferential solvation in mixed solvents. In: Smith PE, Matteoli E, O’Connell JP (eds) Fluctuation theory of solutions: applications in chemistry, chemical engineering, and biophysics. CRC Press, Boca Raton, pp 65–92
Marcus Y (1990) Solubility and solvation in mixed solvent systems. Pure Appl Chem 62:2069–2076
Marcus Y (1998) The properties of solvents. Wiley, Chichester
Marcus Y (2006) Preferential solvation in mixed solvents, 14. Mixtures of 1,4-dioxane with organic solvents: Kirkwood-Buff integrals and volume-corrected preferential solvation parameters. J Mol Liq 128:115–126
Marcus Y (2008) On the preferential solvation of drugs and PAHs in binary solvent mixtures. J Mol Liq 140:61–67
Martínez F, Jouyban A, Acree WE Jr (2016) Preferential solvation of etoricoxib in some aqueous binary co-solvent mixtures at 298.15 K. Phys Chem Liq 55:291–303
Martínez F, Jouyban A, Acree WE Jr (2017) Solubility of phenobarbital in aqueous co-solvent mixtures revisited: IKBI preferential solvation analysis. Phys Chem Liq 55:432–443
Medina L, Bueno JL (2001) Solubilities of zopiclone and nimodipine in supercritical carbon dioxide. J Chem Eng Data 46:1211–1214
Nagata I, Yamada T, Nakagawa S (1975) Excess Gibbs free energies and heats of mixing for binary systems ethyl acetate with methanol, ethanol, 1-propanol, and 2-propanol. J Chem Eng Data 20:271–275
Noubigh A, Akermi A (2017) Solubility and thermodynamic behavior of syringic acid in eight pure and water + methanol mixed solvents. J Chem Eng Data 62:3274–3283
Noubigh A, Akremi A (2019) Experimental measurements, equilibrium study and model correlation of methyl paraben in ethanol and methanol aqueous solutions from = (293.15 to 323.15) K. J Mol Liq 293:111505
Noubigh A (2019) Stearic acid solubility in mixed solvents of (water + ethanol) and (ethanol+ ethyl acetate): experimental data and comparison among different thermodynamic models. J Mol Liq 296:112101
Noubigh A, Abderrabba M (2022) Thermodynamic modeling of the solubility and preferential solvation of the natural product vanillic acid in some aqueous mixtures of alcohols at diferent temperatures. J Chem Eng Data 67:2675–2686
Noubigh A, Abualreish MJ, Mogharbel RT, Alanazi AF, Alanazi TS, Guetat A (2023a) Solubility measurement of syringic acid, trans-cinnamic acid, p-coumaric acid, and vanillin constituent of Deverra DC. genus in (ethyl acetate + ethyl alcohol) mixtures: thermodynamic modeling and preferential solvation. J Mol Liq 387:122710
Noubigh A, Ben Tahar L, Eladeb A (2023b) Solubility modeling and preferential solvation of benzamide in some pure and binary Solvent mixtures at different temperatures. J Chem Eng Data 68:1018–1030
Padervand M, Elahifard MR (2017) Development of a spectrophotometric method for the measurement of kinetic solubility: economical approach to be used in pharmaceutical companies. Pharm Chem J 51:511–515
Padervand M, Teymoorzadeh F, Beheshti A, Bahrami MK (2021a) Preferential solvation of pomalidomide, an immunomodulatory drug, in some biocompatible binary mixed solvents at 298.15 K. Russ J Phys Chem A 95:2432–2443
Padervand M, Naseri S, Hajiahmadi S (2021b) Preferential solvation and solvation shell composition of sunitinib malate, an anti-tumor compound, in some binary mixed solvents at 298.15 K. Chem Pap 75:939–950
Ruidiaz MA, Delgado DR, Martínez F, Marcus Y (2010) Solubility and preferential solvation of indomethacin in 1, 4-dioxane+ water solvent mixtures. Fluid Phase Equilib 299:259–265
Sanders CC (1983) Azlocillin: a new broad spectrum penicillin. J Antimicrob Chemother 11:21–31
Taft RW, Kamlet MJ (1976) The solvatochromic comparison method. II. The alpha-scale of solvent hydrogen-bond donor (HBA) acidities. J Am Chem Soc 98:2886–2894
Tooski HF, Abbari MJ, Farajtabar A (2016) Solubility and preferential solvation of the favonoid naringenin in some aqueous/organic solvent mixtures. J Solut Chem 45:1701–1714
Vahdati S, Shayanfar A, Hanaee J, Martínez F, Acree WE Jr, Jouyban A (2013) Solubility of carvedilol in ethanol + propylene glycol mixtures at various temperatures. Ind Eng Chem Res 52:16630–16636
Vergouwen MD, Vermeulen YB, Roos, (2016) Effect of nimodipine on outcome in patients with traumatic subarachnoid haemorrhage: a systematic review. Lancet Neurol 5:1029–1032
Wang J, Xu AL, Ma ZY (2017) Solubility of amiodarone hydrochloride in aqueous co-Solvent mixtures revisited: IBKI preferential solvation analysis. J Chem Thermodyn 112:276–282
Wu YL, Scott EM, Po ALW, Tariq VN (1999) Ability of azlocillin and tobramycin in combinationto delay or prevent resistance development in Pseudomonas aeruginosa. J Antimicrob Chemother 44:389–392
Yu S, **ng W, Xue F, Cheng Y, Li B (2021) Solubility and thermodynamic properties of nimodipine in pure and binary solvents at a series of temperatures. J Chem Thermodyn 152:106259
Zhang X, Wang Y, Huang X, Meng X, Luo L, Li X, Sakoth B, Hao H (2019) Solubility and thermodynamic properties of azlocillin in pure and binary solvent systems. J Mol Liq 286:110897
Acknowledgements
The authors gratefully acknowledge the approval and the support of this research study by the grant no. SCIA-2023-12-2046 from the Deanship of the Scientific Research in Northern Border University, Arar, KSA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Noubigh, A., Abualreish, M.J. & Tahar, L.B. Solubility and preferential solvation of nimodipine and azlocillin drug compounds in (ethanol + ethyl acetate) mixtures. Braz. J. Chem. Eng. (2024). https://doi.org/10.1007/s43153-024-00458-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43153-024-00458-8