Abstract
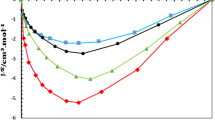
In the current study, the solubility of mesalazine in the binary mixtures of propylene glycol (PG) + 2-propanol was determined using a shake flask method followed by UV-Vis spectroscopy method. The results showed that the maximum solubility was obtained in neat PG (xm = 4.57 × 10− 4) at 313.2 K and the minimum solubility in the neat 2-propanol (xm = 6.32 × 10− 5) at 293.2 K. In the next step, all data were fitted/back-calculated to several mathematical cosolvency models. Deviations of back-calculated data from experimental values (< 4.0%) show the applicability of the models for the prediction of solubility of meselazine in binary mixtures selected. For saturated systems, density data were also determined and fitted to the adopted form of the Jouyban-Acree model (back-calculated MRD% values were within 0.2%). Furthermore, the thermodynamic behavior of mesalazine in the PG + 2-propanol mixtures was investigated using the Gibbs energy, enthalpy, and entropy calculations at Thm.





Similar content being viewed by others
Data avalibility
Data generated or analyzed during this study are provided in full within the published article.
References
Barzegar-Jalali M, Mazaher Haji Agha E, Adibkia K, Hemmati S, Martinez F, Jouyban A (2021) Solubility of mesalazine in {acetonitrile + water} mixtures at various temperatures. Phys Chem Liq 59(5):690–705
Bayan MF, Bayan RF (2020) Recent advances in mesalamine colonic delivery systems. Future J Pharm Sci 6(1):43. https://doi.org/10.1186/s43094-020-00057-7
Bernstein CN, Eaden J, Steinhart AH, Munkholm P, Gordon PH (2002) Cancer prevention in inflammatory bowel disease and the chemoprophylactic potential of 5-aminosalicylic acid. Inflamm Bowel Dis 8(5):356–361. https://doi.org/10.1097/00054725-200209000-00007
Chen C, Liu M, Lii S, Gao C, Chen J (2012) In vitro degradation and drug-release properties of water-soluble chitosan cross-linked oxidized sodium alginate core-shell microgels. J Biomater Sci Polym Ed 23(16):2007–2024. https://doi.org/10.1163/092050611x601720
Dubey R, Dubey R, Omrey P, Vyas SP, Jain SK (2010) Development and characterization of colon specific drug delivery system bearing 5-ASA and Camylofine dihydrochloride for the treatment of ulcerative colitis. J Drug Target 18(8):589–601. https://doi.org/10.3109/10611860903572933
Fiorentini MT, Fracchia M, Galatola G, Barlotta A, De La Pierre M (1990) Acute pancreatitis during oral 5-aminosalicylic acid therapy. Dig Dis Sci 35(9):1180–1182. https://doi.org/10.1007/BF01537594
Grodowska K, Parczewski A (2010) Organic solvents in the pharmaceutical industry. Acta Pol Pharm 67(1):3–12
Hu D, Liu L, Chen W, Li S, Zhao Y (2012) A novel preparation method for 5-aminosalicylic acid loaded Eudragit S100 nanoparticles. Int J Mol Sci 13(5):6454–6468. https://doi.org/10.3390/ijms13056454
Iacucci M, de Silva S, Ghosh S (2010) Mesalazine in inflammatory bowel disease: a trendy topic once again? Can J Gastroenterol 24(2):127–133. https://doi.org/10.1155/2010/586092
Jouyban A (2010) Handbook of Solubility Data for Pharmaceuticals, 1st edn. CRC Press Taylor & Francis Group, Boca Rato
Jouyban A, Acree WE (2021) A single model to represent physico-chemical properties of liquid mixtures at various temperatures. J Mol Liq 323:115054. https://doi.org/10.1016/j.molliq.2020.115054
Jouyban K, Mazaher Haji Agha E, Hemmati S, Martinez F, Kuentz M, Jouyban A (2020) Solubility of 5-aminosalicylic acid in N-methyl-2-pyrrolidone + water mixtures at various temperatures. J Mol Liq 310:113143. https://doi.org/10.1016/j.molliq.2020.113143
Jouyban-Gharamaleki V, Jouyban A, Kuentz M, Hemmati S, Martinez F, Rahimpour E (2020a) A laser monitoring technique for determination of mesalazine solubility in propylene glycol and ethanol mixtures at various temperatures. J Mol Liq 304:112714. https://doi.org/10.1016/j.molliq.2020.112714
Jouyban-Gharamaleki V, Rahimpour E, Hemmati S, Martinez F, Jouyban A (2020b) Mesalazine solubility in propylene glycol and water mixtures at various temperatures using a laser monitoring technique. J Mol Liq 299:112136. https://doi.org/10.1016/j.molliq.2019.112136
Kim IS, Oh IJ (2005) Drug release from the enzyme-degradable and pH-sensitive hydrogel composed of glycidyl methacrylate dextran and poly(acrylic acid). Arch Pharm Res 28(8):983–987. https://doi.org/10.1007/bf02973887
Kim YH, Kim MH, Kim BJ, Kim JJ, Chang DK, Son HJ et al (2009) Inhibition of cell proliferation and invasion in a human colon cancer cell line by 5-aminosalicylic acid. Dig Liver Dis 41(5):328–337. https://doi.org/10.1016/j.dld.2008.09.003
Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK et al (2019) British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 68(Suppl 3):s1–s106. https://doi.org/10.1136/gutjnl-2019-318484
Lau ET, Giddings SJ, Mohammed SG, Dubois P, Johnson SK, Stanley RA et al (2013) Encapsulation of hydrocortisone and mesalazine in zein microparticles. Pharmaceutics 5(2):277–293. https://doi.org/10.3390/pharmaceutics5020277
Mazaher Haji Agha E, Barzegar-Jalali M, Adibkia K, Hemmati S, Kuentz M, Martinez F et al (2020a) Solubility of mesalazine in {1-propanol/water} mixtures at different temperatures. J Mol Liq 301:112436. https://doi.org/10.1016/j.molliq.2019.112436
Mazaher Haji Agha E, Barzegar-Jalali M, Adibkia K, Hemmati S, Martinez F, Jouyban A (2020b) Solubility and thermodynamic properties of mesalazine in {2-propanol + water} mixtures at various temperatures. J Mol Liq 301:112474. https://doi.org/10.1016/j.molliq.2020.112474
Mohammadian E, Foroumadi A, Hasanvand Z, Rahimpour E, Zhao H, Jouyban A (2022) Simulation of mesalazine solubility in the binary solvents at various temperatures. J Mol Liq 357:119160. https://doi.org/10.1016/j.molliq.2022.119160
Moradi M, Mazaher Haji Agha E, Hemmati S, Martinez F, Kuentz M, Jouyban A (2020) Solubility of 5-aminosalicylic acid in {N-methyl-2-pyrrolidone + ethanol} mixtures at T = (293.2 to 313.2) K. J Mol Liq 306:112774. https://doi.org/10.1016/j.molliq.2020.112774
Murray A, Nguyen TM, Parker CE, Feagan BG, MacDonald JK (2020) Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev 8(8):Cd000544. https://doi.org/10.1002/14651858.CD000544.pub5
Nugrahani I, Jessica MA (2021) Amino acids as the potential Co-Former for Co-Crystal Development: a review. Molecules 26(11). https://doi.org/10.3390/molecules26113279
Nunthanid J, Luangtana-anan M, Sriamornsak P, Limmatvapirat S, Huanbutta K, Puttipipatkhachorn S (2009) Use of spray-dried chitosan acetate and ethylcellulose as compression coats for colonic drug delivery: Effect of swelling on triggering in vitro drug release. Eur J Pharm Biopharm 71(2):356–361. https://doi.org/10.1016/j.ejpb.2008.08.002
Pantelic I, Lukic M, Markovic B, Lusiana, Hoffmann C, Müller-Goymann C et al (2014) Development of a prospective isopropyl alcohol-loaded pharmaceutical base using simultaneous in vitro/in vivo characterization methods of skin performance. Drug Dev Ind Pharm 40(7):960–971. https://doi.org/10.3109/03639045.2013.794827
Perlovich GL, Kurkov SV, Bauer-Brandl A (2003) Thermodynamics of solutions. II. Flurbiprofen and diflunisal as models for studying solvation of drug substances. Eur J Pharm Sci 19(5):423–432. https://doi.org/10.1016/s0928-0987(03)00145-3
Rezaei H, Jouyban A, Martinez F, Barzegar-Jalali M, Hemmati S, Rahimpour E (2021) Solubility and thermodynamic profile of mesalazine in carbitol + ethanol mixtures at different temperatures. J Mol Liq 324:114763. https://doi.org/10.1016/j.molliq.2020.114763
Sheikhi-Sovari A, Jouyban A, Martinez F, Hemmati S, Rahimpour E (2021) Solubility of mesalazine in ethylene glycol + water mixtures at different temperatures. J Mol Liq 323:114597. https://doi.org/10.1016/j.molliq.2020.114597
Smetanová L, Stětinová V, Kholová D, Kuneš M, Nobilis M, Svoboda Z et al (2013) Transintestinal transport mechanisms of 5-aminosalicylic acid (in situ rat intestine perfusion, Caco-2 cells) and Biopharmaceutics classification system. Gen Physiol Biophys 32(3):361–369. https://doi.org/10.4149/gpb_2013034
Tang P, Sun Q, Zhao L, Pu H, Yang H, Zhang S et al (2018) Mesalazine/hydroxypropyl-β-cyclodextrin/chitosan nanoparticles with sustained release and enhanced anti-inflammation activity. Carbohydr Polym 198:418–425. https://doi.org/10.1016/j.carbpol.2018.06.106
Vahdati S, Shayanfar A, Hanaee J, Martínez F, Acree WE Jr, Jouyban A (2013) Solubility of carvedilol in ethanol + propylene glycol mixtures at various temperatures. Ind Eng Chem Res 52(47):16630–16636
Velayos FS, Terdiman JP, Walsh JM (2005) Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: a systematic review and metaanalysis of observational studies. Am J Gastroenterol 100(6):1345–1353. https://doi.org/10.1111/j.1572-0241.2005.41442.x
Zarghampour A, Moradi M, Martinez F, Hemmati S, Rahimpour E, Jouyban A (2021) Solubility study of mesalazine in the aqueous mixtures of a deep-eutectic solvent at different temperatures. J Mol Liq 336:116300. https://doi.org/10.1016/j.molliq.2021.116300
Funding
This work was supported by Research Affairs of Tabriz University of Medical Sciences, under Grant number 67898.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Poturcu, K., Zarghampour, A., Rahimpour, E. et al. Solubility and thermodynamic study of mesalazine in propylene glycol + 2-propanol mixtures. Braz. J. Chem. Eng. 40, 1227–1238 (2023). https://doi.org/10.1007/s43153-023-00306-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-023-00306-1