Abstract
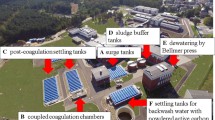
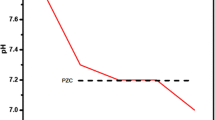
The excess of fluoride ions in potable water is an important public health problem. This study has evaluated the thermal treatment process of a sludge from a water treatment plant at five different temperatures (200, 300, 400, 500, and 600 °C) to find a low-cost and eco-friendly adsorbent for fluoride removal. The sludge characterization was evaluated by thermogravimetric analysis, point of zero charge, scanning electron microscopy coupled with energy dispersive spectroscopy, X-ray diffraction, Fourier transform infrared spectroscopy, and N2 physisorption analysis, aiming to identify changes caused by the thermal treatment and its impact on F− adsorption. All the thermally treated samples were submitted to the same operational conditions in an adsorption experiment and compared with the raw sample. The thermally treated sludge showed the best adsorbent performance at 200 °C. By the use of this adsorbent material, the fluoride removal percentage was 98.13%, and the adsorption capacity was 1.05 mg g−1, resulting in a final fluoride concentration close to zero and meeting the recommendation of the World Health Organization (WHO). These results indicate that the thermal treatment of a water treatment plant sludge could be viable. On one side, thermal treatment is an option to treat problematic waste (sludge) generated in large amounts. On the other hand, this thermally treated sludge can be used as a largely available, accessible, eco-friendly, and low-cost adsorbent for fluoride removal from aqueous media.








Similar content being viewed by others
References
Ahamad KU, Singh R, Baruah I, Choudhury H, Sharma MR (2018) Equilibrium and kinetics modeling of fluoride adsorption onto activated alumina, alum and brick powder. Groundw Sustain Dev 7:452–458
Ahmad T, Ahmad K, Ahad A, Alam M (2016a) Characterization of water treatment sludge and its reuse as coagulant. J Environ Manag 182:606–611
Ahmad T, Ahmad K, Alam M (2016b) Sustainable management of water treatment sludge through 3‘R’ concept. J Clean Prod 124:1–13
Babatunde AO, Zhao YQ, Yang Y, Kearney P (2008) Reuse of dewatered aluminium-coagulated water treatment residual to immobilize phosphorus: batch and column trials using a condensed phosphate. Chem Eng J 136(2–3):108–115
Barathi M, Kumar ASK, Rajesh N (2019) Impact of fluoride in potable water - an outlook on the existing defluoridation strategies and the road ahead. Coord Chem Rev 387:121–128
Bhaumik R, Mondal NK, Das B, Roy P, Pal KC, Das C, Baneerjee A, Datta JK (2012) Eggshell powder as an adsorbent for removal of fluoride from aqueous solution: equilibrium, kinetic and thermodynamic studies. EJ Chem 9:1457–1480
Duarte AL, Da Boit K, Oliveira MLS, Teixeira EC, Schneider IL, Silva LFO (2019) Hazardous elements and amorphous nanoparticles in historical estuary coal mining area. Geosci Front 10:927–939
Dwivedi AD, Dubey SP, Gopal K, Tandon VK (2010) A comparative investigation for strengthening the adsorptive phenomenon by activated natural minerals and plant waste-carbon for defluoridation in water milieu. Desalination 263:189–199
Gao M, Wang W, Yang H, Ye BC (2020) Efficient removal of fluoride from aqueous solutions using 3D flower-like hierarchical zinc-magnesium-aluminum ternary oxide microspheres. Chem Eng J 380:122459
Geng W, Nakajima T, Takanashi H, Ohki A (2009) Analysis of carboxyl group in coal and coal aromaticity by Fourier transform infrared (FT-IR) spectrometry. Fuel 88:139–144
Jangkorn S, Kuhakaew S, Theantanoo S, Klinla-or H, Sriwiriyarat T (2011) Evaluation of reusing alum sludge for the coagulation of industrial wastewater containing mixed anionic surfactants. J Environ Sci 23:587–594
Jeon EK, Ryu S, Park SW, Wang L, Tsang DCW, Baek K (2018) Enhanced adsorption of arsenic onto alum sludge modified by calcination. J Clean Prod 176:54–62
Kazi TG, Brahman KD, Baig JA, Afrid HI (2018) A new efficient indigenous material for simultaneous removal of fluoride and inorganic arsenic species from groundwater. J Hazard Mater 357:159–167
Kumari S, Khan S (2018) Effect of Fe3O4 NPs application on fluoride (F) accumulation efficiency of Prosopis juliflora. Ecotoxicol Environ Saf 166:419–426
León-Mejía G, Machado MN, Okuro RT, Silva LF, Telles C, Dias J, Niekraszewicz L, Da Silva J, Henriques JAP, Zin WA (2018) Intratracheal instillation of coal and coal fly ash particles in mice induces dna damage and translocation of metals to extrapulmonary tissues. Sci Total Environ 625:589–599
Leyva-Ramos R, Rivera-Utrilla J, Medellin-Castillo NA, Sanchez-Polo M (2010) Kinetic modeling of fluoride adsorption from aqueous solution onto bone char. Chem Eng J 158:458–467
Loganathan P, Vigneswarana S, Kandasamy J, Naidu R (2013) Defluoridation of drinking water using adsorption processes. J Hazard Mater 248–249:1–19
Maraschin M, Ferrari KFSH, Silva APH, Carissimi E (2020) Aluminum sludge thickening: Novel helical pipes for aggregation by dual flocculation and thickening by filtration applied to water treatment plants. Sep Purif Technol 24:116560
Mohammad A, Majumder CB (2014) Removal of fluoride from synthetic waste water by using “bio-adsorbents.” Int J Res Eng Technol 3:776–785
Mohan D, Sharma R, Singh VK, Steele P, Pittman CU Jr (2012) Fluoride removal from water using bio-char, a green waste, low-cost adsorbent: equilibrium uptake and sorption dynamics modeling. Ind Eng Chem Res 51:900–914
Sebdani MM, Mauro JC, Jensen LR, Smedskjaer MM (2015) Structure-property relations in calcium aluminate glasses containing different divalent cations and SiO2. J Noncryst Solid 427:160–165
Singh S, German M, Chaudhari S, Sengupta AK (2020) Fluoride removal from groundwater using zirconium impregnated anion exchange resin. J Environ Manag 263:110415
Siswoyo E, Qoniah I, Lestari P, Fajri JA, Sani RA, Sari DG, Boving T (2019) Development of a floating adsorbent for cadmium derived from modified drinking water treatment plant sludge. Environ Technol Innov 14:100312
Tantawy MA (2015) Characterization and pozzolanic properties of calcined alum sludge. Mater Res Bull 61:415–421
Teh CY, Budiman PM, Shak KPY, Wu TY (2016) Recent advancement of coagulation-flocculation and its application in wastewater treatment. Ind Eng Chem Res 55:4363–4389
Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, Sing KSW (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem 87:1051–1069
Tor A, Danaoglu N, Arslan G, Cengeloglu Y (2009) Removal of fluoride from water by using granular red mud: batch and column studies. J Hazard Mater 164:271–278
WHO (1998) Guidelines for drinking water quality, 2nd edn. Addendum to health criteria and other supporting information. World health organization, Geneva, vol 2, pp 123–152
**ngbin S, Chengju X, Zhaochao H (2010) The fluoride-adsorption capacity and influencing factors study of Zeolite. Int Conf Chall Environ Sci Comput Eng 1:358–361
Xu L, Chen G, Peng C, Qiao H, Ke F, Hou R, Li D, Cai H, Wan X (2017) Adsorptive removal of fluoride from drinking water using porous starch loaded with common metal ions. Carbohydr Polym 160:82–89
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pigatto, R.S., Somavilla, E.A., Carissimi, É. et al. Thermally treated sludge obtained from a coagulation–flocculation water treatment process as a low-cost and eco-friendly adsorbent for water defluorination. Braz. J. Chem. Eng. 38, 451–460 (2021). https://doi.org/10.1007/s43153-021-00117-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-021-00117-2